Diabetes aggravates myocardial ischaemia reperfusion injury via activating Nox2-related programmed cell death in an AMPK-dependent manner
- PMID: 32351005
- PMCID: PMC7299688
- DOI: 10.1111/jcmm.15318
Diabetes aggravates myocardial ischaemia reperfusion injury via activating Nox2-related programmed cell death in an AMPK-dependent manner
Abstract
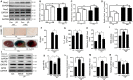
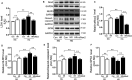
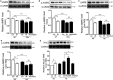
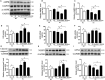
Cardiovascular diseases such as myocardial ischaemia have a high fatality rate in patients with diabetes. This study was designed to expose the crosstalk between oxidative stress and AMPK, a vital molecule that controls biological energy metabolism, in myocardial ischaemia reperfusion injury (I/RI) in diabetic rats. Diabetes was stimulated in rats using streptozotocin injection. Rats were separated on random into control, control + I/R, Diabetes, Diabetes + I/R, Diabetes + I/R + N-acetylcysteine and Diabetes + I/R + Vas2870 groups. Myocardial infarct size was determined, and the predominant Nox family isoforms were analysed. In vitro, the H9C2 cells were administered excess glucose and exposed to hypoxia/reoxygenation to mimic diabetes and I/R. The AMPK siRNA or AICAR was used to inhibit or activate AMPK expression in H9C2 cells, respectively. Then, myocardial oxidative stress and programmed cell death were measured. Diabetes or high glucose levels were found to aggravate myocardial I/RI or hypoxia/reoxygenation in H9C2 cells, as demonstrated by an increase in myocardial infarct size or lactate dehydrogenase levels, oxidative stress generation and induction of programmed cell death. In diabetic rat hearts, cardiac Nox1, Nox2 and Nox4 were all heightened. The suppression of Nox2 expression using Vas2870 or Nox2-siRNA treatment in vivo or in vitro, respectively, protected diabetic rats from myocardial I/RI. AMPK gene knockout increased Nox2 protein expression while AMPK agonist decreased Nox2 expression. Therefore, diabetes aggravates myocardial I/RI by generating of Nox2-associated oxidative stress in an AMPK-dependent manner, which led to the induction of programmed cell death such as apoptosis, pyroptosis and ferroptosis.
Keywords: AMPK; Nox2; diabetes; myocardial ischaemia reperfusion injury; programmed cell death.
© 2020 The Authors. Journal of Cellular and Molecular Medicine published by Foundation for Cellular and Molecular Medicine and John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

Similar articles
-
AMPK Contributes to Cardioprotective Effects of Pterostilbene Against Myocardial Ischemia- Reperfusion Injury in Diabetic Rats by Suppressing Cardiac Oxidative Stress and Apoptosis.Cell Physiol Biochem. 2018;46(4):1381-1397. doi: 10.1159/000489154. Epub 2018 Apr 18. Cell Physiol Biochem. 2018. PMID: 29689567
-
Maternal diabetes up-regulates NOX2 and enhances myocardial ischaemia/reperfusion injury in adult offspring.J Cell Mol Med. 2018 Apr;22(4):2200-2209. doi: 10.1111/jcmm.13500. Epub 2018 Jan 29. J Cell Mol Med. 2018. PMID: 29377505 Free PMC article.
-
Cardiac sodium-dependent glucose cotransporter 1 is a novel mediator of ischaemia/reperfusion injury.Cardiovasc Res. 2019 Sep 1;115(11):1646-1658. doi: 10.1093/cvr/cvz037. Cardiovasc Res. 2019. PMID: 30715251 Free PMC article.
-
Berberine reduces ischemia/reperfusion-induced myocardial apoptosis via activating AMPK and PI3K-Akt signaling in diabetic rats.Apoptosis. 2014 Jun;19(6):946-57. doi: 10.1007/s10495-014-0977-0. Apoptosis. 2014. PMID: 24664781
-
Physiological and pathological functions of NADPH oxidases during myocardial ischemia-reperfusion.Trends Cardiovasc Med. 2014 Jul;24(5):202-5. doi: 10.1016/j.tcm.2014.03.003. Epub 2014 Apr 1. Trends Cardiovasc Med. 2014. PMID: 24880746 Free PMC article. Review.
Cited by
-
NADPH Dynamics: Linking Insulin Resistance and β-Cells Ferroptosis in Diabetes Mellitus.Int J Mol Sci. 2023 Dec 26;25(1):342. doi: 10.3390/ijms25010342. Int J Mol Sci. 2023. PMID: 38203517 Free PMC article. Review.
-
Molecular Mechanisms of Ferroptosis and Relevance to Cardiovascular Disease.Cells. 2022 Sep 1;11(17):2726. doi: 10.3390/cells11172726. Cells. 2022. PMID: 36078133 Free PMC article. Review.
-
Interplay of Oxidative Stress and Necrosis-like Cell Death in Cardiac Ischemia/Reperfusion Injury: A Focus on Necroptosis.Biomedicines. 2022 Jan 7;10(1):127. doi: 10.3390/biomedicines10010127. Biomedicines. 2022. PMID: 35052807 Free PMC article. Review.
-
Ferroptosis inhibition attenuates inflammatory response in mice with acute hypertriglyceridemic pancreatitis.World J Gastroenterol. 2023 Apr 21;29(15):2294-2309. doi: 10.3748/wjg.v29.i15.2294. World J Gastroenterol. 2023. PMID: 37124891 Free PMC article.
-
Emerging roles of ferroptosis in cardiovascular diseases.Cell Death Discov. 2022 Sep 20;8(1):394. doi: 10.1038/s41420-022-01183-2. Cell Death Discov. 2022. PMID: 36127318 Free PMC article. Review.
References
-
- Writing Group M, Mozaffarian D, Benjamin EJ, et al. Executive summary: heart disease and stroke statistics–2016 update: a report from the American Heart Association. Circulation. 2016;133(4):447‐454. - PubMed
-
- Palomer X, Salvado L, Barroso E, Vazquez‐Carrera M. An overview of the crosstalk between inflammatory processes and metabolic dysregulation during diabetic cardiomyopathy. Int J Cardiol. 2013;168(4):3160‐3172. - PubMed
-
- Xia Z, Li H, Irwin MG. Myocardial ischaemia reperfusion injury: the challenge of translating ischaemic and anaesthetic protection from animal models to humans, Br J Anaesth. 2016;117(Suppl 2): ii44‐ii62. - PubMed
-
- Li Y, Zhou ZH, Chen MH, et al. Inhibition of mitochondrial fission and NOX2 expression prevent NLRP3 inflammasome activation in the endothelium: the role of corosolic acid action in the amelioration of endothelial dysfunction. Antioxid Redox Signal. 2016;24(16):893‐908. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

