Draft genome sequences of Hirudo medicinalis and salivary transcriptome of three closely related medicinal leeches
- PMID: 32349672
- PMCID: PMC7191736
- DOI: 10.1186/s12864-020-6748-0
Draft genome sequences of Hirudo medicinalis and salivary transcriptome of three closely related medicinal leeches
Erratum in
-
Correction to: Draft genome sequences of Hirudo medicinalis and salivary transcriptome of three closely related medicinal leeches.BMC Genomics. 2020 Jul 22;21(1):503. doi: 10.1186/s12864-020-06897-0. BMC Genomics. 2020. PMID: 32698877 Free PMC article.
Abstract
Background: Salivary cell secretion (SCS) plays a critical role in blood feeding by medicinal leeches, making them of use for certain medical purposes even today.
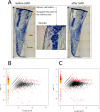
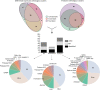
Results: We annotated the Hirudo medicinalis genome and performed RNA-seq on salivary cells isolated from three closely related leech species, H. medicinalis, Hirudo orientalis, and Hirudo verbana. Differential expression analysis verified by proteomics identified salivary cell-specific gene expression, many of which encode previously unknown salivary components. However, the genes encoding known anticoagulants have been found to be expressed not only in salivary cells. The function-related analysis of the unique salivary cell genes enabled an update of the concept of interactions between salivary proteins and components of haemostasis.
Conclusions: Here we report a genome draft of Hirudo medicinalis and describe identification of novel salivary proteins and new homologs of genes encoding known anticoagulants in transcriptomes of three medicinal leech species. Our data provide new insights in genetics of blood-feeding lifestyle in leeches.
Keywords: Anticoagulants; Genome; Haematophagy; Leech H. medicinalis; Systems biology.
Conflict of interest statement
The authors declare no competing financial interests.
Figures

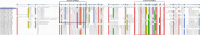



Similar articles
-
Draft genome of the European medicinal leech Hirudo medicinalis (Annelida, Clitellata, Hirudiniformes) with emphasis on anticoagulants.Sci Rep. 2020 Jun 18;10(1):9885. doi: 10.1038/s41598-020-66749-5. Sci Rep. 2020. PMID: 32555498 Free PMC article.
-
Transcriptomic profiling and the first spatial expression analysis of candidate genes in the salivary gland of the East Asian medicinal leech, Hirudo nipponia.Dev Comp Immunol. 2024 May;154:105125. doi: 10.1016/j.dci.2023.105125. Epub 2023 Dec 28. Dev Comp Immunol. 2024. PMID: 38158145
-
Proteins and peptides of the salivary gland secretion of medicinal leeches Hirudo verbana, H. medicinalis, and H. orientalis.Biochemistry (Mosc). 2008 Mar;73(3):315-20. doi: 10.1134/s0006297908030127. Biochemistry (Mosc). 2008. PMID: 18393768
-
Small bite, large impact-saliva and salivary molecules in the medicinal leech, Hirudo medicinalis.Naturwissenschaften. 2011 Dec;98(12):995-1008. doi: 10.1007/s00114-011-0859-z. Epub 2011 Nov 9. Naturwissenschaften. 2011. PMID: 22069059 Review.
-
[Medicinal leeches and hirudotherapy].Turkiye Parazitol Derg. 2011;35(4):234-9. doi: 10.5152/tpd.2011.60. Turkiye Parazitol Derg. 2011. PMID: 22198928 Review. Turkish.
Cited by
-
The genome of medicinal leech (Whitmania pigra) and comparative genomic study for exploration of bioactive ingredients.BMC Genomics. 2022 Jan 24;23(1):76. doi: 10.1186/s12864-022-08290-5. BMC Genomics. 2022. PMID: 35073842 Free PMC article.
-
Macrobdella decora: Old World Leech Gut Microbial Community Structure Conserved in a New World Leech.Appl Environ Microbiol. 2021 Apr 27;87(10):e02082-20. doi: 10.1128/AEM.02082-20. Print 2021 Apr 27. Appl Environ Microbiol. 2021. PMID: 33674439 Free PMC article.
-
A Chromosome-Level Genome Assembly of the Non-Hematophagous Leech Whitmania pigra (Whitman 1884): Identification and Expression Analysis of Antithrombotic Genes.Genes (Basel). 2024 Jan 26;15(2):164. doi: 10.3390/genes15020164. Genes (Basel). 2024. PMID: 38397154 Free PMC article.
-
Acceleration of genome rearrangement in clitellate annelids.bioRxiv [Preprint]. 2024 May 14:2024.05.12.593736. doi: 10.1101/2024.05.12.593736. bioRxiv. 2024. PMID: 38798472 Free PMC article. Preprint.
-
Correction to: Draft genome sequences of Hirudo medicinalis and salivary transcriptome of three closely related medicinal leeches.BMC Genomics. 2020 Jul 22;21(1):503. doi: 10.1186/s12864-020-06897-0. BMC Genomics. 2020. PMID: 32698877 Free PMC article.
References
-
- Mesquita RD, Vionette-Amaral RJ, Lowenberger C, Rivera-Pomar R, Monteiro FA, Minx P, et al. Genome of Rhodnius prolixus , an insect vector of Chagas disease, reveals unique adaptations to hematophagy and parasite infection. Proc Natl Acad Sci. 2015;112:14936–14941. doi: 10.1073/pnas.1506226112. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

