Centromeric cohesion failure invokes a conserved choreography of chromosomal mis-segregations in pancreatic neuroendocrine tumor
- PMID: 32345369
- PMCID: PMC7189550
- DOI: 10.1186/s13073-020-00730-9
Centromeric cohesion failure invokes a conserved choreography of chromosomal mis-segregations in pancreatic neuroendocrine tumor
Abstract
Background: Pancreatic neuroendocrine tumors (PANETs) are rare, slow growing cancers that often present with local and distant metastasis upon detection. PANETS contain distinct karyotypes, epigenetic dysregulation, and recurrent mutations in MEN1, ATRX, and DAXX (MAD+); however, the molecular basis of disease progression remains uncharacterized.
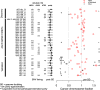
Methods: We evaluated associations between aneuploidy and the MAD+ mutational state of 532 PANETs from 11 published genomic studies and 19 new cases using a combination of exome, targeted panel, shallow WGS, or RNA-seq. We mapped the molecular timing of MAD+ PANET progression using cellular fractions corrected for inferred tumor content.
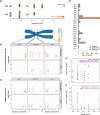
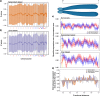
Results: In 287 PANETs with mutational data, MAD+ tumors always exhibited a highly recurrent signature of loss of heterozygosity (LOH) and copy-number alterations affecting 11 chromosomes, typically followed by genome doubling upon metastasis. These LOH chromosomes substantially overlap with those that undergo non-random mis-segregation due to ectopic CENP-A localization to flanking centromeric regions in DAXX-depleted cell lines. Using expression data from 122 PANETs, we found decreased gene expression in the regions immediately adjacent to the centromere in MAD+ PANETs. Using 43 PANETs from AACR GENIE, we inferred this signature to be preceded by mutations in MEN1, ATRX, and DAXX. We conducted a meta-analysis on 226 PANETs from 8 CGH studies to show an association of this signature with metastatic incidence. Our study shows that MAD+ tumors are a genetically diverse and aggressive subtype of PANETs that display extensive chromosomal loss after MAD+ mutation, which is followed by genome doubling.
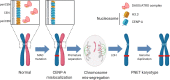
Conclusions: We propose an evolutionary model for a subset of aggressive PANETs that is initiated by mutation of MEN1, ATRX, and DAXX, resulting in defects in centromere cohesion from ectopic CENP-A deposition that leads to selective loss of chromosomes and the LOH phenotype seen in late-stage metastatic PANETs. These insights aid in disease risk stratification and nominate potential therapeutic vulnerabilities to treat this disease.
Keywords: Exome sequencing; Gene expression profiling; Genetic instability; Loss of heterozygosity; Molecular cytogenetics; Molecular timing; Pancreatic neuroendocrine tumors; Publicly available data; Whole-genome sequencing.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


Similar articles
-
MEN1/DAXX/ATRX mutations enhance progression-free survival in gastroenteropancreatic neuroendocrine tumors treated with peptide receptor radionuclide therapy.Endocr Relat Cancer. 2024 Oct 4;31(11):e240065. doi: 10.1530/ERC-24-0065. Print 2024 Nov 1. Endocr Relat Cancer. 2024. PMID: 39093924
-
Prognostic Significance of Altered ATRX/DAXX Gene in Pancreatic Neuroendocrine Tumors: A Meta-Analysis.Front Endocrinol (Lausanne). 2021 Jun 18;12:691557. doi: 10.3389/fendo.2021.691557. eCollection 2021. Front Endocrinol (Lausanne). 2021. PMID: 34220718 Free PMC article.
-
Alternative Lengthening of Telomeres and Loss of DAXX/ATRX Expression Predicts Metastatic Disease and Poor Survival in Patients with Pancreatic Neuroendocrine Tumors.Clin Cancer Res. 2017 Jan 15;23(2):600-609. doi: 10.1158/1078-0432.CCR-16-1113. Epub 2016 Jul 12. Clin Cancer Res. 2017. PMID: 27407094 Free PMC article.
-
Alternative Lengthening of Telomeres (ALT) in Pancreatic Neuroendocrine Tumors: Ready for Prime-Time in Clinical Practice?Curr Oncol Rep. 2021 Jul 16;23(9):106. doi: 10.1007/s11912-021-01096-w. Curr Oncol Rep. 2021. PMID: 34269919 Free PMC article. Review.
-
Prognostic and Predictive Biomarkers for Pancreatic Neuroendocrine Tumors.Surg Pathol Clin. 2022 Sep;15(3):541-554. doi: 10.1016/j.path.2022.05.007. Epub 2022 Aug 2. Surg Pathol Clin. 2022. PMID: 36049835 Review.
Cited by
-
RSF1 in cancer: interactions and functions.Cancer Cell Int. 2021 Jun 19;21(1):315. doi: 10.1186/s12935-021-02012-9. Cancer Cell Int. 2021. PMID: 34147108 Free PMC article. Review.
-
Well-differentiated G1 and G2 pancreatic neuroendocrine tumors: a meta-analysis of published expanded DNA sequencing data.Front Endocrinol (Lausanne). 2024 May 29;15:1351624. doi: 10.3389/fendo.2024.1351624. eCollection 2024. Front Endocrinol (Lausanne). 2024. PMID: 38868744 Free PMC article.
-
CENP-A is a potential prognostic biomarker and correlated with immune infiltration levels in glioma patients.Front Genet. 2022 Aug 29;13:931222. doi: 10.3389/fgene.2022.931222. eCollection 2022. Front Genet. 2022. PMID: 36105094 Free PMC article.
-
Alternative lengthening of telomeres: mechanism and the pathogenesis of cancer.J Clin Pathol. 2024 Jan 18;77(2):82-86. doi: 10.1136/jcp-2023-209005. J Clin Pathol. 2024. PMID: 37890990 Free PMC article.
-
DAXX promotes centromeric stability independently of ATRX by preventing the accumulation of R-loop-induced DNA double-stranded breaks.Nucleic Acids Res. 2024 Feb 9;52(3):1136-1155. doi: 10.1093/nar/gkad1141. Nucleic Acids Res. 2024. PMID: 38038252 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

