Effect of High-Dose Topical Minoxidil on Erythrocyte Quality in SKH1 Hairless Mice
- PMID: 32340110
- PMCID: PMC7222831
- DOI: 10.3390/ani10040731
Effect of High-Dose Topical Minoxidil on Erythrocyte Quality in SKH1 Hairless Mice
Abstract
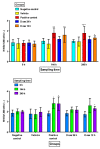
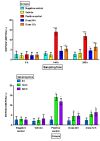
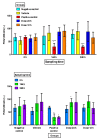
SKH1 hairless mice are widely used in carcinogenesis and dermatology research due to their bare skin, as exposure to different agents is facilitated. Minoxidil is a cosmetic drug that is recognized as a mitogenic agent, and mitogens are suggested to have carcinogenic and mutagenic potential by inducing cell division and increasing the possibility of perpetuating DNA damage. Therefore, we hypothesized that the application of high doses of minoxidil to the skin of hairless mice would increase the number of micronucleated erythrocytes (MNEs) in peripheral blood. The objective of this study was to evaluate the topical administration of high doses of minoxidil on peripheral blood erythrocytes of SKH1 mice by means of micronucleus assay. Minoxidil was administered on the entire body surface of mice every 12 or 24 h. Minoxidil dosing every 24 h increased the number of micronucleated polychromatic erythrocytes (MNPCEs), and dosing every 12 h increased the number of MNEs and MNPCEs, as compared to baseline and the negative control group. No decrease in polychromatic erythrocyte frequencies was observed in the minoxidil groups. Therefore, topical application of high minoxidil doses to mice can produce DNA damage, as observed through an increase in the number of MNEs, without producing cytotoxicity, possibly due to its mitogenic effect.
Keywords: DNA damage; genotoxicity; hairless mice; micronuclei; minoxidil; mitogenic agent.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures
Similar articles
-
Genotoxic evaluation of pirfenidone using erythrocyte rodent micronucleus assay.Food Chem Toxicol. 2012 Aug;50(8):2760-5. doi: 10.1016/j.fct.2012.05.049. Epub 2012 Jun 7. Food Chem Toxicol. 2012. PMID: 22683486
-
Micronucleated erythrocyte frequencies in old and new world primates: measurement of micronucleated erythrocyte frequencies in peripheral blood of Callithrix jacchus as a model for evaluating genotoxicity in primates.Environ Mol Mutagen. 2005 Dec;46(4):253-9. doi: 10.1002/em.20154. Environ Mol Mutagen. 2005. PMID: 15971258
-
Quantitative and qualitative studies of micronucleus induction in mouse erythrocytes using flow cytometry. I. Measurement of micronucleus induction in peripheral blood polychromatic erythrocytes by chemicals with known and suspected genotoxicity.Mutagenesis. 1997 Jan;12(1):1-8. doi: 10.1093/mutage/12.1.1. Mutagenesis. 1997. PMID: 9025090
-
Toxicology and carcinogenesis studies of androstenedione (CAS No. 63-05-8) in F344/N rats and B6C3F1 mice (gavage studies).Natl Toxicol Program Tech Rep Ser. 2010 Sep;(560):1, 7-31,33-171 passim. Natl Toxicol Program Tech Rep Ser. 2010. PMID: 21037592 Review.
-
Final report on the safety assessment of capsicum annuum extract, capsicum annuum fruit extract, capsicum annuum resin, capsicum annuum fruit powder, capsicum frutescens fruit, capsicum frutescens fruit extract, capsicum frutescens resin, and capsaicin.Int J Toxicol. 2007;26 Suppl 1:3-106. doi: 10.1080/10915810601163939. Int J Toxicol. 2007. PMID: 17365137 Review.
References
-
- Gómez-Meda B.C., Zamora-Perez A., Zúñiga-González G.M. Genotoxicity and Biomonitoring: Micronuclei in Peripheral Blood and Epithelial Cells. In: Suzuki A., Kimura A., editors. New Research on DNA Damage. Nova Science Publishers, Inc.; New York, NY, USA: 2008. pp. 145–182.
-
- Alcántar-Díaz B.E., Gómez-Meda B.C., Zúñiga-González G.M., Zamora-Perez A.L., González-Cuevas J., Álvarez-Rodríguez B.A., Sánchez-Parada M.G., García-Bañuelos J.J., Armendáriz-Borunda J. Genotoxic Evaluation of Pirfenidone using Erythrocyte Rodent Micronucleus Assay. Food Chem. Toxicol. 2012;50:2760–2765. doi: 10.1016/j.fct.2012.05.049. - DOI - PubMed
-
- Gómez-Meda B.C., Bañales-Martínez L.R., Zamora-Perez A.L., Lemus-Varela M.L., Trujillo X., Sánchez-Parada M.G., Torres-Mendoza B.M., Armendáriz-Borunda J., Zúñiga-González G.M. Micronucleated Erythrocytes in Peripheral Blood from Neonate Rats Exposed by Breastfeeding to Cyclophosphamide, Colchicine, or Cytosine-Arabinoside. Biomed. Res. Int. 2016;2016:9161648. doi: 10.1155/2016/9161648. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

