PLGA microsphere-based composite hydrogel for dual delivery of ciprofloxacin and ginsenoside Rh2 to treat Staphylococcus aureus-induced skin infections
- PMID: 32329376
- PMCID: PMC7241502
- DOI: 10.1080/10717544.2020.1756985
PLGA microsphere-based composite hydrogel for dual delivery of ciprofloxacin and ginsenoside Rh2 to treat Staphylococcus aureus-induced skin infections
Abstract
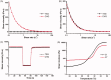
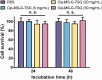
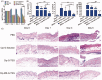
When antibiotic-resistant pathogenic bacteria pose a high threat to human health, bacterial multidrug efflux pumps become major contributors to the high-level antibiotic resistance in most microorganisms. Since traditional antibiotics are still indispensable currently, we report a dual drug delivery system to maximize the antibacterial efficacy of antibiotics by inhibiting efflux pumps in bacteria before their exposure to antibiotics. In this research, a microsphere/hydrogel composite was constructed from ciprofloxacin (Cip)-loaded poly (lactic-co-glycolic acid) (PLGA) microspheres and ginsenoside Rh2 (G-Rh2) dispersed thermo-sensitive hydrogel to treat skin infections. In vitro drug release studies indicated that while G-Rh2 in hydrogel presented a faster and short-term release manner to rapidly inhibit the NorA efflux pumps, Cip showed a sustained and long-term release behavior to provide a local high concentration gradient for facilitating drug percutaneous penetration. The combination of Cip and G-Rh2 demonstrated a high degree of synergism against both methicillin-sensitive Staphylococcus aureus (MSSA) and methicillin-resistant Staphylococcus aureus (MRSA), hence significantly improving their in vitro antibacterial activity and efficiency. Moreover, the antibacterial performance of the microsphere/hydrogel composite with a sequential release profile is superior to that of other formulations in mouse model of MRSA skin infections, indicating its great potential to treat antibiotic-resistant skin infections.
Keywords: Ciprofloxacin; ginsenoside Rh2; microsphere/hydrogel composite; sequential release; skin infections.
Figures

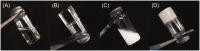


Similar articles
-
Non-antibiotic agent ginsenoside 20(S)-Rh2 enhanced the antibacterial effects of ciprofloxacin in vitro and in vivo as a potential NorA inhibitor.Eur J Pharmacol. 2014 Oct 5;740:277-84. doi: 10.1016/j.ejphar.2014.07.020. Epub 2014 Jul 20. Eur J Pharmacol. 2014. PMID: 25054686
-
PLGA Nanoparticles Formulations Loaded With Antibiotics Induce Sustained and Controlled Antibiotics Release for Prolonged Antibacterial Action Against MRSA, and Pseudomonas aeruginosa FRD1.Mil Med. 2024 Aug 19;189(Suppl 3):230-238. doi: 10.1093/milmed/usae079. Mil Med. 2024. PMID: 39160825
-
Near-infrared light-controllable on-demand antibiotics release using thermo-sensitive hydrogel-based drug reservoir for combating bacterial infection.Biomaterials. 2019 Jan;188:83-95. doi: 10.1016/j.biomaterials.2018.09.045. Epub 2018 Oct 2. Biomaterials. 2019. PMID: 30339942
-
Current and emerging drugs for acute bacterial skin and skin structure infections: an update.Expert Opin Emerg Drugs. 2014 Sep;19(3):431-40. doi: 10.1517/14728214.2014.955015. Epub 2014 Aug 22. Expert Opin Emerg Drugs. 2014. PMID: 25146459 Review.
-
Nano delivery systems to the rescue of ciprofloxacin against resistant bacteria "E. coli; P. aeruginosa; Saureus; and MRSA" and their infections.J Control Release. 2022 Sep;349:338-353. doi: 10.1016/j.jconrel.2022.07.003. Epub 2022 Jul 12. J Control Release. 2022. PMID: 35820538 Review.
Cited by
-
Effect of anti-skin disorders of ginsenosides- A Systematic Review.J Ginseng Res. 2023 Sep;47(5):605-614. doi: 10.1016/j.jgr.2023.04.005. Epub 2023 May 4. J Ginseng Res. 2023. PMID: 37720567 Free PMC article. Review.
-
Biofunctionalization of Poly(lactide-co-glycolic acid) Using Potent NorA Efflux Pump Inhibitors Immobilized on Nanometric Alpha-Zirconium Phosphate to Reduce Biofilm Formation.Materials (Basel). 2021 Feb 1;14(3):670. doi: 10.3390/ma14030670. Materials (Basel). 2021. PMID: 33535577 Free PMC article.
-
Membrane-disruptive peptides/peptidomimetics-based therapeutics: Promising systems to combat bacteria and cancer in the drug-resistant era.Acta Pharm Sin B. 2021 Sep;11(9):2609-2644. doi: 10.1016/j.apsb.2021.07.014. Epub 2021 Jul 21. Acta Pharm Sin B. 2021. PMID: 34589385 Free PMC article. Review.
-
Influence of Auricularia cornea Polysaccharide Coating on the Stability and Antioxidant Activity of Liposomes Ginsenoside Rh2.Foods. 2023 Oct 29;12(21):3946. doi: 10.3390/foods12213946. Foods. 2023. PMID: 37959065 Free PMC article.
-
Polymer based dual drug delivery system for targeted treatment of fluoroquinolone resistant Staphylococcus aureus mediated infections.Sci Rep. 2023 Jul 14;13(1):11373. doi: 10.1038/s41598-023-38473-3. Sci Rep. 2023. PMID: 37452106 Free PMC article.
References
-
- Årdal C, Balasegaram M, Laxminarayan R, et al. (2019). Antibiotic development—economic, regulatory and societal challenges. Nat Rev Microbiol.18:267–74. - PubMed
-
- Astolfi A, Felicetti T, Iraci N, et al. (2017). Pharmacophore-based repositioning of approved drugs as novel Staphylococcus aureus NorA efflux pump inhibitors. J Med Chem 60:1598–604. - PubMed
-
- Cao XX, Ye QL, Fan MW, et al. (2019). Antimicrobial effects of the ginsenoside Rh2 on monospecies and multispecies cariogenic biofilms. J Appl Microbiol 126:740–51. - PubMed
-
- Cebrián L, Rodríguez JC, Escribano I, et al. (2005). Characterization of Salmonella spp. Mutants with reduced fluoroquinolone susceptibility: importance of efflux pump mechanisms. Chemotherapy 51:40–3. - PubMed
-
- Deepika MS, Thangam R, Sakthidhasan P, et al. (2018). Combined effect of a natural flavonoid rutin from Citrus sinensis and conventional antibiotic gentamicin on Pseudomonas aeruginosa biofilm formation. Food Control 90:282–94.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
