Spectrin couples cell shape, cortical tension, and Hippo signaling in retinal epithelial morphogenesis
- PMID: 32328630
- PMCID: PMC7147103
- DOI: 10.1083/jcb.201907018
Spectrin couples cell shape, cortical tension, and Hippo signaling in retinal epithelial morphogenesis
Abstract
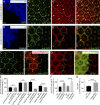
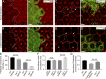
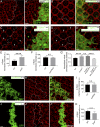
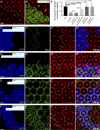
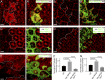
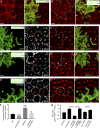
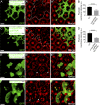
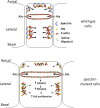
Although extracellular force has a profound effect on cell shape, cytoskeleton tension, and cell proliferation through the Hippo signaling effector Yki/YAP/TAZ, how intracellular force regulates these processes remains poorly understood. Here, we report an essential role for spectrin in specifying cell shape by transmitting intracellular actomyosin force to cell membrane. While activation of myosin II in Drosophila melanogaster pupal retina leads to increased cortical tension, apical constriction, and Yki-mediated hyperplasia, spectrin mutant cells, despite showing myosin II activation and Yki-mediated hyperplasia, paradoxically display decreased cortical tension and expanded apical area. Mechanistically, we show that spectrin is required for tethering cortical F-actin to cell membrane domains outside the adherens junctions (AJs). Thus, in the absence of spectrin, the weakened attachment of cortical F-actin to plasma membrane results in a failure to transmit actomyosin force to cell membrane, causing an expansion of apical surfaces. These results uncover an essential mechanism that couples cell shape, cortical tension, and Hippo signaling and highlight the importance of non-AJ membrane domains in dictating cell shape in tissue morphogenesis.
© 2020 Deng et al.
Figures
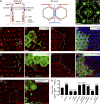
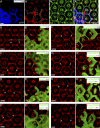
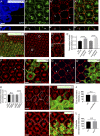
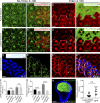

Similar articles
-
Spectrin regulates Hippo signaling by modulating cortical actomyosin activity.Elife. 2015 Mar 31;4:e06567. doi: 10.7554/eLife.06567. Elife. 2015. PMID: 25826608 Free PMC article.
-
Integration of contractile forces during tissue invagination.J Cell Biol. 2010 Mar 8;188(5):735-49. doi: 10.1083/jcb.200910099. Epub 2010 Mar 1. J Cell Biol. 2010. PMID: 20194639 Free PMC article.
-
Competition between myosin II and βH-spectrin regulates cytoskeletal tension.Elife. 2023 Jun 27;12:RP84918. doi: 10.7554/eLife.84918. Elife. 2023. PMID: 37367948 Free PMC article.
-
Integration of cell-cell adhesion and contractile actomyosin activity during morphogenesis.Curr Top Dev Biol. 2015;112:103-27. doi: 10.1016/bs.ctdb.2014.11.017. Epub 2015 Feb 12. Curr Top Dev Biol. 2015. PMID: 25733139 Review.
-
Spectrin-based skeleton as an actor in cell signaling.Cell Mol Life Sci. 2012 Jan;69(2):191-201. doi: 10.1007/s00018-011-0804-5. Epub 2011 Aug 30. Cell Mol Life Sci. 2012. PMID: 21877118 Free PMC article. Review.
Cited by
-
Myotubularin functions through actomyosin to interact with the Hippo pathway.EMBO Rep. 2022 Dec 6;23(12):e55851. doi: 10.15252/embr.202255851. Epub 2022 Oct 26. EMBO Rep. 2022. PMID: 36285521 Free PMC article.
-
Phase separation of Hippo signalling complexes.EMBO J. 2023 Mar 15;42(6):e112863. doi: 10.15252/embj.2022112863. Epub 2023 Feb 20. EMBO J. 2023. PMID: 36807601 Free PMC article.
-
Crumbs and the apical spectrin cytoskeleton regulate R8 cell fate in the Drosophila eye.PLoS Genet. 2021 Jun 7;17(6):e1009146. doi: 10.1371/journal.pgen.1009146. eCollection 2021 Jun. PLoS Genet. 2021. PMID: 34097697 Free PMC article.
-
Cortical tension promotes Kibra degradation via Par-1.Mol Biol Cell. 2024 Jan 1;35(1):ar2. doi: 10.1091/mbc.E23-06-0246. Epub 2023 Oct 30. Mol Biol Cell. 2024. PMID: 37903240 Free PMC article.
-
Stiff matrix induced srGAP2 tension gradients control migration direction in triple-negative breast cancer.Theranostics. 2023 Jan 1;13(1):59-76. doi: 10.7150/thno.77313. eCollection 2023. Theranostics. 2023. PMID: 36593959 Free PMC article.
References
-
- Bennett V., Gardner K., and Steiner J.P.. 1988. Brain adducin: a protein kinase C substrate that may mediate site-directed assembly at the spectrin-actin junction. J. Biol. Chem. 263:5860–5869. - PubMed
-
- Benz P.M., Merkel C.J., Offner K., Abeßer M., Ullrich M., Fischer T., Bayer B., Wagner H., Gambaryan S., Ursitti J.A., et al. . 2013. Mena/VASP and αII-Spectrin complexes regulate cytoplasmic actin networks in cardiomyocytes and protect from conduction abnormalities and dilated cardiomyopathy. Cell Commun. Signal. 11:56 10.1186/1478-811X-11-56 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

