Role of Divalent Cations in HIV-1 Replication and Pathogenicity
- PMID: 32326317
- PMCID: PMC7232465
- DOI: 10.3390/v12040471
Role of Divalent Cations in HIV-1 Replication and Pathogenicity
Abstract
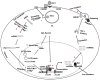
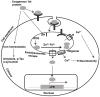
Divalent cations are essential for life and are fundamentally important coordinators of cellular metabolism, cell growth, host-pathogen interactions, and cell death. Specifically, for human immunodeficiency virus type-1 (HIV-1), divalent cations are required for interactions between viral and host factors that govern HIV-1 replication and pathogenicity. Homeostatic regulation of divalent cations' levels and actions appear to change as HIV-1 infection progresses and as changes occur between HIV-1 and the host. In people living with HIV-1, dietary supplementation with divalent cations may increase HIV-1 replication, whereas cation chelation may suppress HIV-1 replication and decrease disease progression. Here, we review literature on the roles of zinc (Zn2+), iron (Fe2+), manganese (Mn2+), magnesium (Mg2+), selenium (Se2+), and copper (Cu2+) in HIV-1 replication and pathogenicity, as well as evidence that divalent cation levels and actions may be targeted therapeutically in people living with HIV-1.
Keywords: HIV-1 associated neurocognitive disorders; divalent cations; endolysosomes; human immunodeficiency virus type-1; transactivator of transcription.
Conflict of interest statement
The authors disclose that this manuscript was written without any commercial or financial associations that could be construed as a conflict of interest.
Figures
Similar articles
-
Endolysosome iron restricts Tat-mediated HIV-1 LTR transactivation by increasing HIV-1 Tat oligomerization and β-catenin expression.J Neurovirol. 2021 Oct;27(5):755-773. doi: 10.1007/s13365-021-01016-5. Epub 2021 Sep 22. J Neurovirol. 2021. PMID: 34550543 Free PMC article.
-
Tat is a multifunctional viral protein that modulates cellular gene expression and functions.Oncotarget. 2017 Apr 18;8(16):27569-27581. doi: 10.18632/oncotarget.15174. Oncotarget. 2017. PMID: 28187438 Free PMC article. Review.
-
Role of divalent metals in infectious disease susceptibility and outcome.Clin Microbiol Infect. 2018 Jan;24(1):16-23. doi: 10.1016/j.cmi.2017.01.018. Epub 2017 Jan 29. Clin Microbiol Infect. 2018. PMID: 28143784 Review.
-
HIV-1 Tat phosphorylation on Ser-16 residue modulates HIV-1 transcription.Retrovirology. 2018 May 23;15(1):39. doi: 10.1186/s12977-018-0422-5. Retrovirology. 2018. PMID: 29792216 Free PMC article.
-
HIV-1 Tat Protein Enters Dysfunctional Endothelial Cells via Integrins and Renders Them Permissive to Virus Replication.Int J Mol Sci. 2020 Dec 30;22(1):317. doi: 10.3390/ijms22010317. Int J Mol Sci. 2020. PMID: 33396807 Free PMC article.
Cited by
-
Endolysosome iron restricts Tat-mediated HIV-1 LTR transactivation by increasing HIV-1 Tat oligomerization and β-catenin expression.J Neurovirol. 2021 Oct;27(5):755-773. doi: 10.1007/s13365-021-01016-5. Epub 2021 Sep 22. J Neurovirol. 2021. PMID: 34550543 Free PMC article.
-
Therapeutic Potential of Iron Chelators in Viral Diseases: A Systematic Review.Curr Med Chem. 2024;31(27):4383-4391. doi: 10.2174/0109298673259596231211113211. Curr Med Chem. 2024. PMID: 38321902
-
Understanding the relationship between viral infections and trace elements from a metallomics perspective: implications for COVID-19.Metallomics. 2020 Dec 23;12(12):1912-1930. doi: 10.1039/d0mt00220h. Metallomics. 2020. PMID: 33295922 Free PMC article. Review.
-
Inhibitory effect and mechanism of gelatin stabilized ferrous sulfide nanoparticles on porcine reproductive and respiratory syndrome virus.J Nanobiotechnology. 2022 Feb 5;20(1):70. doi: 10.1186/s12951-022-01281-4. J Nanobiotechnology. 2022. PMID: 35123507 Free PMC article.
-
Allopregnanolone and neuroHIV: Potential benefits of neuroendocrine modulation in the era of antiretroviral therapy.J Neuroendocrinol. 2022 Feb;34(2):e13047. doi: 10.1111/jne.13047. Epub 2021 Oct 14. J Neuroendocrinol. 2022. PMID: 34651359 Free PMC article. Review.
References
-
- Anastassopoulou J., Theophanides T. The Role of Metal Ions in Biological Systems and Medicine. In: Kessissoglou D.P., editor. Bioinorganic Chemistry: An Inorganic Perspective of Life. Springer; Dordrecht, The Netherlands: 1995. pp. 209–2018.
-
- Sigel A., Sigel H., Sigel R.K.O. Interactions Between Essential Metal Ions and Human Diseases. Springer; Dordrecht, The Netherlands: 2013.
-
- Tan X. Metalloproteins and Metalloenzymes: Roles and Mechanisms of Metals in Functional Proteins. World Scientific Publishing Company Pte Limited; Singapore: 2018.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

