Composition, Predicted Functions and Co-occurrence Networks of Rhizobacterial Communities Impacting Flowering Desert Events in the Atacama Desert, Chile
- PMID: 32322245
- PMCID: PMC7156552
- DOI: 10.3389/fmicb.2020.00571
Composition, Predicted Functions and Co-occurrence Networks of Rhizobacterial Communities Impacting Flowering Desert Events in the Atacama Desert, Chile
Abstract
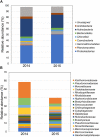
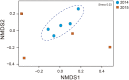
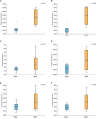
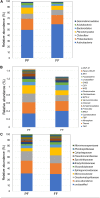
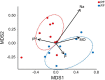
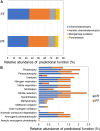
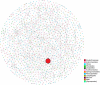
Flowering desert (FD) events consist of the rapid flowering of a wide variety of native plants in the Atacama Desert of Chile, which is categorized as the driest desert in the world. While ephemeral plants are an integral part of the desert ecosystem, there is little knowledge on plant-microbe interactions that occur during FD events. Consequently, the overall goals of this present study were to investigate changes in the composition and potential functions of rhizobacterial community of Cistanthe longiscapa (Montiaceae) during the 2014 and 2015 FD events and determine the composition, potential functions, and co-occurrence networks of rhizobacterial community associated with the root zone of C. longiscapa during pre- (PF) and full-flowering (FF) phenological stages. Results of this study showed that the Proteobacteria and Actinobacteria were the dominant taxa in rhizosphere soils during the three FD events (2014, 2015, and 2017) examined. In general, greater microbial richness and diversity were observed in rhizosphere soils during the 2015-, compared with the 2014-FD event. Similarly, predicted functional analyses indicated that a larger number of sequences were assigned to information processing (e.g., ion channel, transporters and ribosome) and metabolism (e.g., lipids, nitrogen, and sulfur) during 2015 compared with 2014. Despite the lack of significant differences in diversity among PF and FF stages, the combined analysis of rhizobacterial community data, along with data concerning rhizosphere soil properties, evidenced differences among both phenological stages and suggested that sodium is a relevant abiotic factor shaping the rhizosphere. In general, no significant differences in predicted functions (most of them assigned to chemoheterotrophy, magnesium metabolisms, and fermentation) were observed among PF and FF. Co-occurrence analysis revealed the complex rhizobacterial interactions that occur in C. longiscapa during FD, highlighting to Kouleothrixaceae family as keystone taxa. Taken together this study shows that the composition and function of rhizobacteria vary among and during FD events, where some bacterial groups and their activity may influence the growth and flowering of native plants, and therefore, the ecology and trophic webs in Atacama Desert.
Keywords: Atacama Desert; Cistanthe longiscapa; co-occurrence network; flowering desert; high-throughput sequencing; rhizobacterial community.
Copyright © 2020 Astorga-Eló, Zhang, Larama, Stoll, Sadowsky and Jorquera.
Figures

Similar articles
-
Microbiome Dynamics Associated With the Atacama Flowering Desert.Front Microbiol. 2020 Jan 22;10:3160. doi: 10.3389/fmicb.2019.03160. eCollection 2019. Front Microbiol. 2020. PMID: 32038589 Free PMC article.
-
Rhizobacterial Community Structures Associated with Native Plants Grown in Chilean Extreme Environments.Microb Ecol. 2016 Oct;72(3):633-46. doi: 10.1007/s00248-016-0813-x. Epub 2016 Jul 13. Microb Ecol. 2016. PMID: 27406732
-
Fungal and Bacterial Microbiome Associated with the Rhizosphere of Native Plants from the Atacama Desert.Microorganisms. 2020 Feb 4;8(2):209. doi: 10.3390/microorganisms8020209. Microorganisms. 2020. PMID: 32033093 Free PMC article.
-
Fungal diversity in the Atacama Desert.Antonie Van Leeuwenhoek. 2018 Aug;111(8):1345-1360. doi: 10.1007/s10482-018-1060-6. Epub 2018 Mar 7. Antonie Van Leeuwenhoek. 2018. PMID: 29516313 Review.
-
Rare taxa and dark microbial matter: novel bioactive actinobacteria abound in Atacama Desert soils.Antonie Van Leeuwenhoek. 2018 Aug;111(8):1315-1332. doi: 10.1007/s10482-018-1088-7. Epub 2018 May 2. Antonie Van Leeuwenhoek. 2018. PMID: 29721711 Review.
Cited by
-
Rhizobacteria from 'flowering desert' events contribute to the mitigation of water scarcity stress during tomato seedling germination and growth.Sci Rep. 2021 Jul 2;11(1):13745. doi: 10.1038/s41598-021-93303-8. Sci Rep. 2021. PMID: 34215802 Free PMC article.
-
The phytomicrobiome: solving plant stress tolerance under climate change.Front Plant Sci. 2023 Sep 7;14:1219366. doi: 10.3389/fpls.2023.1219366. eCollection 2023. Front Plant Sci. 2023. PMID: 37746004 Free PMC article. Review.
-
Natural Holobiome Engineering by Using Native Extreme Microbiome to Counteract the Climate Change Effects.Front Bioeng Biotechnol. 2020 Jun 4;8:568. doi: 10.3389/fbioe.2020.00568. eCollection 2020. Front Bioeng Biotechnol. 2020. PMID: 32582678 Free PMC article. Review.
-
Nitrogen fixation and other biogeochemically important features of Atacama Desert giant horsetail plant microbiomes inferred from metagenomic contig analysis.Ann Bot. 2022 Jul 19;130(1):65-75. doi: 10.1093/aob/mcac060. Ann Bot. 2022. PMID: 35533355 Free PMC article.
-
Microbiome Variation Across Populations of Desert Halophyte Zygophyllum qatarensis.Front Plant Sci. 2022 Mar 31;13:841217. doi: 10.3389/fpls.2022.841217. eCollection 2022. Front Plant Sci. 2022. PMID: 35432394 Free PMC article.
References
-
- Aguirre-Garrido F., Montiel-Lugo D., Hernández-Rodríguez C., Torres-Cortes G., Millán V., Toro N., et al. (2012). Bacterial community structure in the rhizosphere of three cactus species from semi-arid highlands in central Mexico. Antonie van Leeuwenhoek 101 891–904. 10.1007/s10482-012-9705-3 - DOI - PubMed
-
- Aislabie J. M., Chhour K., Saul D. J., Miyauchi S., Ayton J., Paetzold R. F., et al. (2006). Dominant bacteria in soils of marble point and wright valley, victoria land, Antarctica. Soil Biol. Biochem. 38 3041–3056. 10.1016/j.soilbio.2006.02.018 - DOI
-
- Aronesty E. (2013). Comparison of sequencing utility programs. Open Bioinform. J. 7 1–8. 10.2174/1875036201307010001 - DOI
-
- Assis R. A. B., Polloni L. C., Patané J. S., Thakur S., Felestrino E. B., Diaz-Caballero J., et al. (2017). Identification and analysis of seven effector protein families with different adaptive and evolutionary histories in plant-associated members of the Xanthomonadaceae. Sci. Rep. 7:16433. 10.1038/s41598-017-16325-1 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

