Constitutive and Stress-Induced Psychomotor Cortical Responses to Compound K Supplementation
- PMID: 32322188
- PMCID: PMC7158875
- DOI: 10.3389/fnins.2020.00315
Constitutive and Stress-Induced Psychomotor Cortical Responses to Compound K Supplementation
Abstract
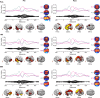
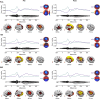
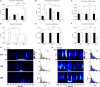
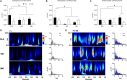
Isolated ginsenoside metabolites such as Compound K (CK) are of increasing interest to consumer and clinical populations as safe and non-pharmacological means to enhance psychomotor performance constitutively and in response to physical or cognitive stress. Nevertheless, the influence of CK on behavioral performance and EEG measures of cortical activity in humans is undetermined. In this double-blinded, placebo-controlled, counterbalanced within-group study, dose-dependent responses to CK (placebo, 160 and 960 mg) were assessed after 2 weeks of supplementation in nineteen healthy men and women (age: 39.9 ± 7.9 year, height 170.2 ± 8.6 cm, weight 79.7 ± 11.9 kg). Performance on upper- and lower-body choice reaction tests (CRTs) was tested before and after intense lower-body anaerobic exercise. Treatment- and stress-related changes in brain activity were measured with high-density EEG based on event-related potentials, oscillations, and source activity. Upper- (-12.3 ± 3.5 ms, p = 0.002) and lower-body (-12.3 ± 4.9 ms, p = 0.021) response times improved after exercise, with no difference between treatments (upper: p = 0.354; lower: p = 0.926). Analysis of cortical activity in sensor and source space revealed global increases in cortical arousal after exercise. CK increased activity in cortical regions responsible for sustained attention and mitigated exercise-induced increases in arousal. Responses to exercise varied depending on task, but CK appeared to reduce sensory interference from lower-body exercise during an upper-body CRT and improve the general maintenance of task-relevant sensory processes.
Keywords: EEG; cortical activity; event-related potentials; exercise; ginsenoside; psychomotor performance; source localization; stress.
Copyright © 2020 Flanagan, Proessl, Dunn-Lewis, Canino, Sterczala, Connaboy, DuPont, Caldwell and Kraemer.
Figures


Similar articles
-
The Effects of a Korean Ginseng, GINST15, on Hypo-Pituitary-Adrenal and Oxidative Activity Induced by Intense Work Stress.J Med Food. 2018 Jan;21(1):104-112. doi: 10.1089/jmf.2017.0071. Epub 2017 Oct 5. J Med Food. 2018. PMID: 28981384 Clinical Trial.
-
Acute exercise induces cortical inhibition and reduces arousal in response to visual stimulation in young children.Int J Dev Neurosci. 2014 May;34:1-8. doi: 10.1016/j.ijdevneu.2013.12.009. Epub 2014 Jan 8. Int J Dev Neurosci. 2014. PMID: 24412583 Clinical Trial.
-
Beneficial effects of acute high-intensity exercise on electrophysiological indices of attention processes in young adult men.Behav Brain Res. 2019 Feb 1;359:474-484. doi: 10.1016/j.bbr.2018.11.024. Epub 2018 Nov 19. Behav Brain Res. 2019. PMID: 30465815 Free PMC article. Clinical Trial.
-
Mapping of the neuronal networks of human cortical brain functions.Adv Tech Stand Neurosurg. 2003;28:91-142. doi: 10.1007/978-3-7091-0641-9_2. Adv Tech Stand Neurosurg. 2003. PMID: 12627809 Review.
-
Behavioral Counseling to Promote a Healthful Diet and Physical Activity for Cardiovascular Disease Prevention in Adults Without Known Cardiovascular Disease Risk Factors: Updated Systematic Review for the U.S. Preventive Services Task Force [Internet].Rockville (MD): Agency for Healthcare Research and Quality (US); 2017 Jul. Report No.: 15-05222-EF-1. Rockville (MD): Agency for Healthcare Research and Quality (US); 2017 Jul. Report No.: 15-05222-EF-1. PMID: 29364620 Free Books & Documents. Review.
Cited by
-
Ginsenoside Compound K: Insights into Recent Studies on Pharmacokinetics and Health-Promoting Activities.Biomolecules. 2020 Jul 10;10(7):1028. doi: 10.3390/biom10071028. Biomolecules. 2020. PMID: 32664389 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources
Research Materials

