Microbiome, bile acids, and obesity: How microbially modified metabolites shape anti-tumor immunity
- PMID: 32320071
- PMCID: PMC7841960
- DOI: 10.1111/imr.12856
Microbiome, bile acids, and obesity: How microbially modified metabolites shape anti-tumor immunity
Abstract
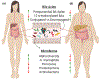
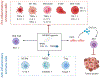
Bile acids (BAs) are known facilitators of nutrient absorption but recent paradigm shifts now recognize BAs as signaling molecules regulating both innate and adaptive immunity. Bile acids are synthesized from cholesterol in the liver with subsequent microbial modification and fermentation adding complexity to pool composition. Bile acids act on several receptors such as Farnesoid X Receptor and the G protein-coupled BA receptor 1 (TGR5). Interestingly, BA receptors (BARs) are expressed on immune cells and activation either by BAs or BAR agonists modulates innate and adaptive immune cell populations skewing their polarization toward a more tolerogenic anti-inflammatory phenotype. Intriguingly, recent evidence also suggests that BAs promote anti-tumor immune response through activation and recruitment of tumoricidal immune cells such as natural killer T cells. These exciting findings have redefined BA signaling in health and disease wherein they may suppress inflammation on the one hand, yet promote anti-tumor immunity on the other hand. In this review, we provide our readers with the most recent understanding of the interaction of BAs with the host microbiome, their effect on innate and adaptive immunity in health and disease with a special focus on obesity, bariatric surgery-induced weight loss, and immune checkpoint blockade in cancer.
Keywords: bariatric surgery; immunometabolism; microbiome; mitochondria; obesity; tumor microenvironment.
© 2020 John Wiley & Sons A/S. Published by John Wiley & Sons Ltd.
Conflict of interest statement
CONFLICT OF INTEREST
No authors have conflict.
Figures
Similar articles
-
The role of bile acids in reducing the metabolic complications of obesity after bariatric surgery: a systematic review.Int J Obes (Lond). 2015 Nov;39(11):1565-74. doi: 10.1038/ijo.2015.115. Epub 2015 Jun 17. Int J Obes (Lond). 2015. PMID: 26081915 Review.
-
Bile Acid-Activated Receptors, Intestinal Microbiota, and the Treatment of Metabolic Disorders.Trends Mol Med. 2015 Nov;21(11):702-714. doi: 10.1016/j.molmed.2015.09.001. Epub 2015 Oct 16. Trends Mol Med. 2015. PMID: 26481828 Review.
-
Signaling from Intestine to the Host: How Bile Acids Regulate Intestinal and Liver Immunity.Handb Exp Pharmacol. 2019;256:95-108. doi: 10.1007/164_2019_225. Handb Exp Pharmacol. 2019. PMID: 31119464 Review.
-
Bile acid is a significant host factor shaping the gut microbiome of diet-induced obese mice.BMC Biol. 2017 Dec 14;15(1):120. doi: 10.1186/s12915-017-0462-7. BMC Biol. 2017. PMID: 29241453 Free PMC article.
-
The Role of the Gut Microbiota in Bile Acid Metabolism.Ann Hepatol. 2017 Nov;16(Suppl. 1: s3-105.):s15-s20. doi: 10.5604/01.3001.0010.5494. Ann Hepatol. 2017. PMID: 29080339 Review.
Cited by
-
Multi-omics analyses reveal that the gut microbiome and its metabolites promote milk fat synthesis in Zhongdian yak cows.PeerJ. 2022 Dec 2;10:e14444. doi: 10.7717/peerj.14444. eCollection 2022. PeerJ. 2022. PMID: 36518262 Free PMC article.
-
Metabolism, metabolites, and macrophages in cancer.J Hematol Oncol. 2023 Jul 25;16(1):80. doi: 10.1186/s13045-023-01478-6. J Hematol Oncol. 2023. PMID: 37491279 Free PMC article. Review.
-
Bile Acids: Physiological Activity and Perspectives of Using in Clinical and Laboratory Diagnostics.Molecules. 2022 Nov 13;27(22):7830. doi: 10.3390/molecules27227830. Molecules. 2022. PMID: 36431930 Free PMC article. Review.
-
Obesity, dysbiosis and inflammation: interactions that modulate the efficacy of immunotherapy.Front Immunol. 2024 Aug 26;15:1444589. doi: 10.3389/fimmu.2024.1444589. eCollection 2024. Front Immunol. 2024. PMID: 39253073 Free PMC article. Review.
-
Microbiota-dependent regulation of costimulatory and coinhibitory pathways via innate immune sensors and implications for immunotherapy.Exp Mol Med. 2023 Sep;55(9):1913-1921. doi: 10.1038/s12276-023-01075-0. Epub 2023 Sep 11. Exp Mol Med. 2023. PMID: 37696895 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

