Nystatin Regulates Axonal Extension and Regeneration by Modifying the Levels of Nitric Oxide
- PMID: 32317932
- PMCID: PMC7146717
- DOI: 10.3389/fnmol.2020.00056
Nystatin Regulates Axonal Extension and Regeneration by Modifying the Levels of Nitric Oxide
Abstract
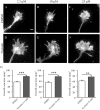
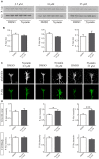
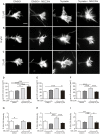
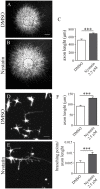
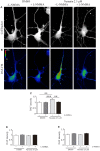
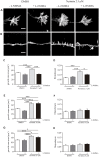
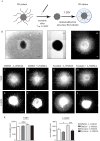
Nystatin is a pharmacological agent commonly used for the treatment of oral, mucosal and cutaneous fungal infections. Nystatin has also been extensively applied to study the cellular function of cholesterol-enriched structures because of its ability to bind and extract cholesterol from mammalian membranes. In neurons, cholesterol level is tightly regulated, being essential for synapse and dendrite formation, and axonal guidance. However, the action of Nystatin on axon regeneration has been poorly evaluated. Here, we examine the effect of Nystatin on primary cultures of hippocampal neurons, showing how acute dose (minutes) of Nystatin increases the area of growth cones, and chronic treatment (days) enhances axon length, axon branching, and axon regeneration post-axotomy. We describe two alternative signaling pathways responsible for the observed effects and activated at different concentrations of Nystatin. At elevated concentrations, Nystatin promotes growth cone expansion through phosphorylation of Akt; whereas, at low concentrations, Nystatin enhances axon length and regrowth by increasing nitric oxide levels. Together, our findings indicate new signaling pathways of Nystatin and propose this compound as a novel regulator of axon regeneration.
Keywords: axon growth; axon regrowth post-axotomy; growth cone; nitric oxide synthase; nystatin.
Copyright © 2020 Roselló-Busquets, Hernaiz-Llorens, Soriano and Martínez-Mármol.
Figures
Similar articles
-
Cholesterol Depletion Regulates Axonal Growth and Enhances Central and Peripheral Nerve Regeneration.Front Cell Neurosci. 2019 Feb 12;13:40. doi: 10.3389/fncel.2019.00040. eCollection 2019. Front Cell Neurosci. 2019. PMID: 30809129 Free PMC article.
-
Akt1-Inhibitor of DNA binding2 is essential for growth cone formation and axon growth and promotes central nervous system axon regeneration.Elife. 2016 Dec 12;5:e20799. doi: 10.7554/eLife.20799. Elife. 2016. PMID: 27938661 Free PMC article.
-
PI3K/Akt and ERK/MAPK Signaling Promote Different Aspects of Neuron Survival and Axonal Regrowth Following Rat Facial Nerve Axotomy.Neurochem Res. 2017 Dec;42(12):3515-3524. doi: 10.1007/s11064-017-2399-1. Epub 2017 Oct 9. Neurochem Res. 2017. PMID: 28993995
-
Cell death and axon regeneration of Purkinje cells after axotomy: challenges of classical hypotheses of axon regeneration.Brain Res Brain Res Rev. 2005 Sep;49(2):300-16. doi: 10.1016/j.brainresrev.2004.11.007. Epub 2005 Mar 2. Brain Res Brain Res Rev. 2005. PMID: 16111558 Review.
-
Membrane trafficking events underlying axon repair, growth, and regeneration.Mol Cell Neurosci. 2011 Dec;48(4):339-48. doi: 10.1016/j.mcn.2011.04.003. Epub 2011 Apr 23. Mol Cell Neurosci. 2011. PMID: 21539917 Review.
Cited by
-
Cholesterol synthesis inhibition or depletion in axon regeneration.Neural Regen Res. 2022 Feb;17(2):271-276. doi: 10.4103/1673-5374.317956. Neural Regen Res. 2022. PMID: 34269187 Free PMC article. Review.
-
Inhibition of the AP-1/TFPI2 axis contributes to alleviating cerebral ischemia/reperfusion injury by improving blood-brain barrier integrity.Hum Cell. 2024 Nov;37(6):1679-1695. doi: 10.1007/s13577-024-01125-3. Epub 2024 Sep 4. Hum Cell. 2024. PMID: 39227518
-
One Raft to Guide Them All, and in Axon Regeneration Inhibit Them.Int J Mol Sci. 2021 May 8;22(9):5009. doi: 10.3390/ijms22095009. Int J Mol Sci. 2021. PMID: 34066896 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources

