A Cdc42-mediated supracellular network drives polarized forces and Drosophila egg chamber extension
- PMID: 32317641
- PMCID: PMC7174421
- DOI: 10.1038/s41467-020-15593-2
A Cdc42-mediated supracellular network drives polarized forces and Drosophila egg chamber extension
Abstract
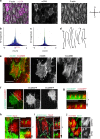
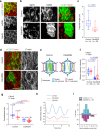
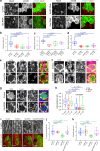
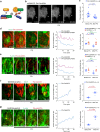
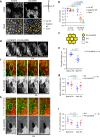
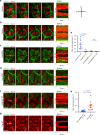
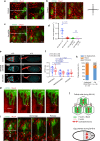
Actomyosin supracellular networks emerge during development and tissue repair. These cytoskeletal structures are able to generate large scale forces that can extensively remodel epithelia driving tissue buckling, closure and extension. How supracellular networks emerge, are controlled and mechanically work still remain elusive. During Drosophila oogenesis, the egg chamber elongates along the anterior-posterior axis. Here we show that a dorsal-ventral polarized supracellular F-actin network, running around the egg chamber on the basal side of follicle cells, emerges from polarized intercellular filopodia that radiate from basal stress fibers and extend penetrating neighboring cell cortexes. Filopodia can be mechanosensitive and function as cell-cell anchoring sites. The small GTPase Cdc42 governs the formation and distribution of intercellular filopodia and stress fibers in follicle cells. Finally, our study shows that a Cdc42-dependent supracellular cytoskeletal network provides a scaffold integrating local oscillatory actomyosin contractions at the tissue scale to drive global polarized forces and tissue elongation.
Conflict of interest statement
The authors declare no competing interests.
Figures
Similar articles
-
Cellular and Supracellular Planar Polarity: A Multiscale Cue to Elongate the Drosophila Egg Chamber.Front Cell Dev Biol. 2021 Mar 2;9:645235. doi: 10.3389/fcell.2021.645235. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 33738289 Free PMC article. Review.
-
Integration of contractile forces during tissue invagination.J Cell Biol. 2010 Mar 8;188(5):735-49. doi: 10.1083/jcb.200910099. Epub 2010 Mar 1. J Cell Biol. 2010. PMID: 20194639 Free PMC article.
-
Drosophila egg chamber elongation: insights into how tissues and organs are shaped.Fly (Austin). 2012 Oct-Dec;6(4):213-27. doi: 10.4161/fly.21969. Epub 2012 Aug 31. Fly (Austin). 2012. PMID: 22940759 Free PMC article. Review.
-
Myosin light-chain phosphatase regulates basal actomyosin oscillations during morphogenesis.Nat Commun. 2016 Feb 18;7:10746. doi: 10.1038/ncomms10746. Nat Commun. 2016. PMID: 26888436 Free PMC article.
-
Planar polarized actomyosin contractile flows control epithelial junction remodelling.Nature. 2010 Dec 23;468(7327):1110-4. doi: 10.1038/nature09566. Epub 2010 Nov 10. Nature. 2010. PMID: 21068726
Cited by
-
Cellular and Supracellular Planar Polarity: A Multiscale Cue to Elongate the Drosophila Egg Chamber.Front Cell Dev Biol. 2021 Mar 2;9:645235. doi: 10.3389/fcell.2021.645235. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 33738289 Free PMC article. Review.
-
Finishing the egg.Genetics. 2024 Jan 3;226(1):iyad183. doi: 10.1093/genetics/iyad183. Genetics. 2024. PMID: 38000906 Free PMC article.
-
Discovery of an F-actin-binding small molecule serving as a fluorescent probe and a scaffold for functional probes.Sci Adv. 2021 Nov 19;7(47):eabg8585. doi: 10.1126/sciadv.abg8585. Epub 2021 Nov 19. Sci Adv. 2021. PMID: 34797716 Free PMC article.
-
Lateral adherens junctions mediate a supracellular actomyosin cortex in drosophila trachea.iScience. 2023 Mar 13;26(4):106380. doi: 10.1016/j.isci.2023.106380. eCollection 2023 Apr 21. iScience. 2023. PMID: 37009223 Free PMC article.
-
The emergence of spontaneous coordinated epithelial rotation on cylindrical curved surfaces.Sci Adv. 2022 Sep 16;8(37):eabn5406. doi: 10.1126/sciadv.abn5406. Epub 2022 Sep 14. Sci Adv. 2022. PMID: 36103541 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

