The TIPE Molecular Pilot That Directs Lymphocyte Migration in Health and Inflammation
- PMID: 32313148
- PMCID: PMC7170861
- DOI: 10.1038/s41598-020-63629-w
The TIPE Molecular Pilot That Directs Lymphocyte Migration in Health and Inflammation
Abstract
Lymphocytes are some of the most motile cells of vertebrates, constantly navigating through various organ systems. Their specific positioning in the body is delicately controlled by site-specific directional cues such as chemokines. While it has long been suspected that an intrinsic molecular pilot, akin to a ship's pilot, guides lymphocyte navigation, the nature of this pilot is unknown. Here we show that the TIPE (TNF-α-induced protein 8-like) family of proteins pilot lymphocytes by steering them toward chemokines. TIPE proteins are carriers of lipid second messengers. They mediate chemokine-induced local generation of phosphoinositide second messengers, but inhibit global activation of the small GTPase Rac. TIPE-deficient T lymphocytes are completely pilot-less: they are unable to migrate toward chemokines despite their normal ability to move randomly. As a consequence, TIPE-deficient mice have a marked defect in positioning their T lymphocytes to various tissues, both at the steady-state and during inflammation. Thus, TIPE proteins pilot lymphocytes during migration and may be targeted for the treatment of lymphocyte-related disorders.
Conflict of interest statement
Y.H.C. is a member of the advisory boards of Amshenn Co. and Binde Co. None of the other authors have any competing interests to disclose.
Figures
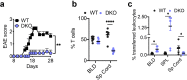
Similar articles
-
TIPE proteins control directed migration of human T cells by directing GPCR and lipid second messenger signaling.J Leukoc Biol. 2024 Feb 23;115(3):511-524. doi: 10.1093/jleuko/qiad141. J Leukoc Biol. 2024. PMID: 37952106 Free PMC article.
-
TNFAIP8 Deficiency Exacerbates Acute Graft Versus Host Disease in a Murine Model of Allogeneic Hematopoietic Cell Transplantation.Transplantation. 2020 Mar;104(3):500-510. doi: 10.1097/TP.0000000000003013. Transplantation. 2020. PMID: 31634333 Free PMC article.
-
Direction of leukocyte polarization and migration by the phosphoinositide-transfer protein TIPE2.Nat Immunol. 2017 Dec;18(12):1353-1360. doi: 10.1038/ni.3866. Epub 2017 Oct 23. Nat Immunol. 2017. PMID: 29058702 Free PMC article.
-
Phosphoinositide 3-kinase-dependent activation of Rac.FEBS Lett. 2003 Jul 3;546(1):93-7. doi: 10.1016/s0014-5793(03)00454-x. FEBS Lett. 2003. PMID: 12829242 Review.
-
TIPE Family of Proteins and Its Implications in Different Chronic Diseases.Int J Mol Sci. 2018 Sep 29;19(10):2974. doi: 10.3390/ijms19102974. Int J Mol Sci. 2018. PMID: 30274259 Free PMC article. Review.
Cited by
-
TIPE proteins control directed migration of human T cells by directing GPCR and lipid second messenger signaling.J Leukoc Biol. 2024 Feb 23;115(3):511-524. doi: 10.1093/jleuko/qiad141. J Leukoc Biol. 2024. PMID: 37952106 Free PMC article.
-
Tnfaip8 and Tipe2 Gene Deletion Ameliorates Immediate Proteoglycan Loss and Inflammatory Responses in the Injured Mouse Intervertebral Disc.Am J Phys Med Rehabil. 2024 Oct 1;103(10):918-924. doi: 10.1097/PHM.0000000000002488. Epub 2024 Apr 8. Am J Phys Med Rehabil. 2024. PMID: 38630557
-
TIPE2 Promotes Tumor Initiation But Inhibits Tumor Progression in Murine Colitis-Associated Colon Cancer.Inflamm Bowel Dis. 2022 May 4;28(5):764-774. doi: 10.1093/ibd/izab306. Inflamm Bowel Dis. 2022. PMID: 34894222 Free PMC article.
-
Decoupling tumor cell metastasis from growth by cellular pilot protein TNFAIP8.Oncogene. 2021 Nov;40(46):6456-6468. doi: 10.1038/s41388-021-02035-6. Epub 2021 Oct 4. Oncogene. 2021. PMID: 34608264 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

