Extracellular S100A4 as a key player in fibrotic diseases
- PMID: 32307910
- PMCID: PMC7294136
- DOI: 10.1111/jcmm.15259
Extracellular S100A4 as a key player in fibrotic diseases
Abstract
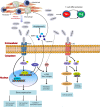
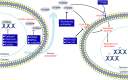
Fibrosis is characterized by fibroblast activation, extracellular matrix (ECM) accumulation and infiltration of inflammatory cells that sometimes leads to irreversible organ dysfunction. Considerable evidence now indicates that inflammation plays a critical role in the initiation and progression of organ fibrosis. S100A4 protein, a ubiquitous member of the S100 family, has recently been discovered as a potential factor implicated in fibrotic diseases. S100A4 protein is released at inflammatory site and has a certain biological function to promote cell motility, invasion, ECM remodelling, autophagy and angiogenesis. In addition, extracellular S100A4 is also a potential causation of inflammatory processes and induces the release of cytokines and growth factors under different pathological conditions. Elevated S100A4 level in patients' serum closely correlates with disease activity in several fibrotic diseases and serves as a useful biomarker for diagnosis and monitoring disease progression. Analyses of knockout mouse models have identified a functional role of extracellular S100A4 protein in fibrotic diseases, suggesting that suppressing its expression, release or function might be a promising therapeutic strategy. This review will focus on the role of extracellular S100A4 as a key regulator of pro-inflammatory signalling pathways and its relative biological processes involved in the pathogenesis of fibrosis.
Keywords: Fibrosis; biomarker; damage-associated molecular pattern; extracellular S100A4; inflammation.
© 2020 The Authors. Journal of Cellular and Molecular Medicine published by Foundation for Cellular and Molecular Medicine and John Wiley & Sons Ltd.
Conflict of interest statement
The authors confirm that there are no conflicts of interest.
Figures
Similar articles
-
The Multifaceted S100A4 Protein in Cancer and Inflammation.Methods Mol Biol. 2019;1929:339-365. doi: 10.1007/978-1-4939-9030-6_22. Methods Mol Biol. 2019. PMID: 30710284 Review.
-
S100A4 in the Physiology and Pathology of the Central and Peripheral Nervous System.Cells. 2021 Apr 2;10(4):798. doi: 10.3390/cells10040798. Cells. 2021. PMID: 33918416 Free PMC article. Review.
-
Clinical significance of serum S100 calcium-binding protein A4 in idiopathic pulmonary fibrosis.Respirology. 2020 Jul;25(7):743-749. doi: 10.1111/resp.13707. Epub 2019 Oct 9. Respirology. 2020. PMID: 31597225
-
The function of S100A4 in pulmonary disease: A review.Medicine (Baltimore). 2023 Apr 7;102(14):e33466. doi: 10.1097/MD.0000000000033466. Medicine (Baltimore). 2023. PMID: 37026957 Free PMC article. Review.
-
S100A4 a classical DAMP as a therapeutic target in fibrosis.Matrix Biol. 2024 Mar;127:1-7. doi: 10.1016/j.matbio.2024.01.002. Epub 2024 Jan 12. Matrix Biol. 2024. PMID: 38219976 Review.
Cited by
-
The miR-124-3p regulates the allergic airway inflammation and remodeling in an ovalbumin-asthmatic mouse model by inhibiting S100A4.Immun Inflamm Dis. 2023 Feb;11(2):e730. doi: 10.1002/iid3.730. Immun Inflamm Dis. 2023. PMID: 36799806 Free PMC article.
-
Histological, Immunohistochemical and Antioxidant Analysis of Skin Wound Healing Influenced by the Topical Application of Brazilian Red Propolis.Antioxidants (Basel). 2022 Nov 4;11(11):2188. doi: 10.3390/antiox11112188. Antioxidants (Basel). 2022. PMID: 36358560 Free PMC article.
-
Protein atlas of fibroblast specific protein 1 (FSP1)/S100A4.Histol Histopathol. 2023 Dec;38(12):1391-1401. doi: 10.14670/HH-18-621. Epub 2023 Apr 13. Histol Histopathol. 2023. PMID: 37154201
-
Fibrosis as a common trait in amyotrophic lateral sclerosis tissues.Neural Regen Res. 2022 Jan;17(1):97-98. doi: 10.4103/1673-5374.314302. Neural Regen Res. 2022. PMID: 34100438 Free PMC article. No abstract available.
-
Enzyme Replacement Therapy for FABRY Disease: Possible Strategies to Improve Its Efficacy.Int J Mol Sci. 2023 Feb 25;24(5):4548. doi: 10.3390/ijms24054548. Int J Mol Sci. 2023. PMID: 36901983 Free PMC article.
References
-
- Feng Y‐L, Chen D‐Q, Vaziri ND, Guo Y, Zhao Y‐Y. Small molecule inhibitors of epithelial‐mesenchymal transition for the treatment of cancer and fibrosis. Med Res Rev. 2020;40:54‐78. - PubMed
-
- Chen DQ, Feng YL, Cao G, Zhao YY. Natural products as a source for antifibrosis therapy. Trends Pharmacol Sci. 2018;39:937‐952. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

