Functionally engineered extracellular vesicles improve bone regeneration
- PMID: 32305445
- PMCID: PMC8040700
- DOI: 10.1016/j.actbio.2020.04.017
Functionally engineered extracellular vesicles improve bone regeneration
Abstract
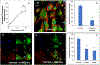
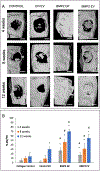
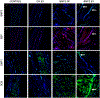
Lineage specific differentiation of host mesenchymal stem cells (MSCs) is a necessary step for bone repair/regeneration. Clinically, growth factors such as bone morphogenetic protein 2 (BMP2) are used to enhance/hasten this process to heal critical sized defects. However, the clinical application of such growth factors is fraught with dosage challenges as well as immunological and ectopic complications. The identification of extracellular vesicles (EVs) as active components of the MSC secretome suggest alternative approaches to enhancing bone regeneration. Based on our earlier studies on the properties of EVs from lineage specified MSCs, this study sought to engineer EVs to enhance osteogenic differentiation. To generate MSC EVs with enhanced osteoinductive abilities, genetically modified human bone marrow derived MSCs (HMSCs) were generated by constitutively expressing BMP2. We hypothesized that these cells would generate functionally engineered EVs (FEEs) with enhanced osteoinductive properties. Our results show that these FEEs maintained the general physical and biochemical characteristics of naïve HMSC EVs in the form of size distribution, EV marker expression and endocytic properties but show increased bone regenerative potential compared to MSC EVs in a rat calvarial defect model in vivo. Mechanistic studies revealed that although BMP2 was constitutively expressed in the parental cells, the corresponding EVs (FEEs) do not contain BMP2 protein as an EV constituent. Further investigations revealed that the FEEs potentiate the BMP2 signaling cascade possibly due to an altered miRNA composition. Collectively, these studies indicate that EVs' functionality may be engineered by genetic modification of the parental MSCs to induce osteoinduction and bone regeneration. SIGNIFICANCE STATEMENT: With mounting evidence for the potential of MSC EVs in treatment of diseases and regeneration of tissues, it is imperative to evaluate if they can be modified for application specificity. The results presented here indicate the possibility for generating Functionally Engineered EVs (FEEs) from MSC sources. As a proof of concept approach, we have shown that EVs derived from genetically modified MSCs (BMP2 overexpression) can be effective as biomimetic substitutes for growth factors for enhanced tissue-specific regeneration (bone regeneration) in vivo. Mechanistic studies highlight the role of EV miRNAs in inducing pathway-specific changes. We believe that this study will be useful to researchers evaluating EVs for regenerative medicine applications.
Keywords: BMP2; Bone regeneration; Exosomes; Extracellular vesicles; Mesenchymal stem cells.
Copyright © 2020 Acta Materialia Inc. Published by Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures



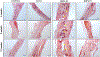
Similar articles
-
Osteogenic human MSC-derived extracellular vesicles regulate MSC activity and osteogenic differentiation and promote bone regeneration in a rat calvarial defect model.Stem Cell Res Ther. 2024 Feb 7;15(1):33. doi: 10.1186/s13287-024-03639-x. Stem Cell Res Ther. 2024. PMID: 38321490 Free PMC article.
-
3D Encapsulation and tethering of functionally engineered extracellular vesicles to hydrogels.Acta Biomater. 2021 May;126:199-210. doi: 10.1016/j.actbio.2021.03.030. Epub 2021 Mar 16. Acta Biomater. 2021. PMID: 33741538 Free PMC article.
-
Bone regeneration is mediated by macrophage extracellular vesicles.Bone. 2020 Dec;141:115627. doi: 10.1016/j.bone.2020.115627. Epub 2020 Sep 3. Bone. 2020. PMID: 32891867 Free PMC article.
-
Extracellular vesicle-loaded hydrogels for tissue repair and regeneration.Mater Today Bio. 2022 Dec 21;18:100522. doi: 10.1016/j.mtbio.2022.100522. eCollection 2023 Feb. Mater Today Bio. 2022. PMID: 36593913 Free PMC article. Review.
-
Mesenchymal stem cell-derived small extracellular vesicles and bone regeneration.Basic Clin Pharmacol Toxicol. 2021 Jan;128(1):18-36. doi: 10.1111/bcpt.13478. Epub 2020 Sep 22. Basic Clin Pharmacol Toxicol. 2021. PMID: 32780530 Free PMC article. Review.
Cited by
-
Safety and potential effects of intrathecal injection of allogeneic human umbilical cord mesenchymal stem cell-derived exosomes in complete subacute spinal cord injury: a first-in-human, single-arm, open-label, phase I clinical trial.Stem Cell Res Ther. 2024 Aug 26;15(1):264. doi: 10.1186/s13287-024-03868-0. Stem Cell Res Ther. 2024. PMID: 39183334 Free PMC article. Clinical Trial.
-
Oral Bone Tissue Regeneration: Mesenchymal Stem Cells, Secretome, and Biomaterials.Int J Mol Sci. 2021 May 15;22(10):5236. doi: 10.3390/ijms22105236. Int J Mol Sci. 2021. PMID: 34063438 Free PMC article. Review.
-
Educating EVs to Improve Bone Regeneration: Getting Closer to the Clinic.Int J Mol Sci. 2022 Feb 7;23(3):1865. doi: 10.3390/ijms23031865. Int J Mol Sci. 2022. PMID: 35163787 Free PMC article. Review.
-
Local delivery of USC-derived exosomes harboring ANGPTL3 enhances spinal cord functional recovery after injury by promoting angiogenesis.Stem Cell Res Ther. 2021 Jan 7;12(1):20. doi: 10.1186/s13287-020-02078-8. Stem Cell Res Ther. 2021. PMID: 33413639 Free PMC article.
-
Mesenchymal Stem Cell-Derived Exosomes as Drug Delivery Vehicles in Disease Therapy.Int J Mol Sci. 2024 Jul 14;25(14):7715. doi: 10.3390/ijms25147715. Int J Mol Sci. 2024. PMID: 39062956 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

