New Insights on the Effect of TNF Alpha Blockade by Gene Silencing in Noise-Induced Hearing Loss
- PMID: 32294929
- PMCID: PMC7215896
- DOI: 10.3390/ijms21082692
New Insights on the Effect of TNF Alpha Blockade by Gene Silencing in Noise-Induced Hearing Loss
Abstract
Noise exposure represents the second most common cause of acquired sensorineural hearing loss and we observed that tumor necrosis factor α (TNFα) was involved in this context. The effect of Tnfα gene silencing on the expression profile related to the TNFα metabolic pathway in an experimental model of noise-induced hearing loss had not previously been studied.
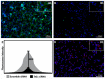
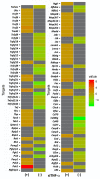
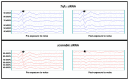
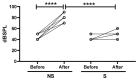
Methods: Single ears of Wistar rats were pretreated with Tnfα small interfering RNA (siRNA) by trans-tympanic administration 24 h before they were exposed to white noise (120 dBSPL for three hours). After 24 h of noise exposure, we analyzed the electrophysiological threshold and the amplitude of waves I, II, III, and IV in the auditory brain response click. In addition, qRT-PCR was performed to evaluate the TNFα metabolic pathway in the ears submitted or not to gene silencing.
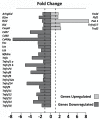
Results: Preservation of the electrophysiological threshold and the amplitude of waves was observed in the ears submitted to gene silencing compared to the ears not treated. Increased anti-apoptotic gene expression and decreased pro-apoptotic gene expression were found in the treated ears.
Conclusion: Our results allow us to suggest that the blockade of TNFα by gene silencing was useful to prevent noise-induced hearing loss.
Keywords: TNFα metabolic pathway; apoptosis; auditory brain response; cochlea; electrophysiological threshold; in vivo siRNA administration; synaptopathy.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Intra-tympanic delivery of short interfering RNA into the adult mouse cochlea.Hear Res. 2013 Feb;296:36-41. doi: 10.1016/j.heares.2012.10.011. Epub 2012 Nov 23. Hear Res. 2013. PMID: 23183031 Free PMC article.
-
Effects of lifetime noise exposure on the middle-age human auditory brainstem response, tinnitus and speech-in-noise intelligibility.Hear Res. 2018 Aug;365:36-48. doi: 10.1016/j.heares.2018.06.003. Epub 2018 Jun 12. Hear Res. 2018. PMID: 29913342
-
Applying Neurotrophins to the Round Window Rescues Auditory Function and Reduces Inner Hair Cell Synaptopathy After Noise-induced Hearing Loss.Otol Neurotol. 2016 Oct;37(9):1223-30. doi: 10.1097/MAO.0000000000001191. Otol Neurotol. 2016. PMID: 27631825
-
[Hidden hearing loss-damage to hearing processing even with low-threshold noise exposure?].HNO. 2019 Jun;67(6):417-424. doi: 10.1007/s00106-019-0640-8. HNO. 2019. PMID: 30874853 Review. German.
-
Speech-in-Noise Tests and Supra-threshold Auditory Evoked Potentials as Metrics for Noise Damage and Clinical Trial Outcome Measures.Otol Neurotol. 2016 Sep;37(8):e295-302. doi: 10.1097/MAO.0000000000001069. Otol Neurotol. 2016. PMID: 27518138 Review.
Cited by
-
Tissue engineering strategies for spiral ganglion neuron protection and regeneration.J Nanobiotechnology. 2024 Jul 31;22(1):458. doi: 10.1186/s12951-024-02742-8. J Nanobiotechnology. 2024. PMID: 39085923 Free PMC article. Review.
-
Recent Therapeutic Progress and Future Perspectives for the Treatment of Hearing Loss.Biomedicines. 2023 Dec 18;11(12):3347. doi: 10.3390/biomedicines11123347. Biomedicines. 2023. PMID: 38137568 Free PMC article. Review.
-
Resveratrol Ameliorates Lipopolysaccharide-Induced Sudden Sensorineural Hearing Loss in In Vitro Model through Multitarget Antiapoptotic Mechanism Based on Network Pharmacology and Molecular Docking.Evid Based Complement Alternat Med. 2022 May 19;2022:6404588. doi: 10.1155/2022/6404588. eCollection 2022. Evid Based Complement Alternat Med. 2022. PMID: 35646137 Free PMC article.
-
Cytomegalovirus Seropositivity as a Potential Risk Factor for Increased Noise Trauma Susceptibility.Noise Health. 2022 Jan-Mar;24(112):1-6. doi: 10.4103/nah.nah_4_21. Noise Health. 2022. PMID: 35645133 Free PMC article.
-
The inflammatory and metabolic status of patients with sudden-onset sensorineural hearing loss.Front Neurol. 2024 Jul 2;15:1382096. doi: 10.3389/fneur.2024.1382096. eCollection 2024. Front Neurol. 2024. PMID: 39015324 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

