Spatial control of membrane traffic in neuronal dendrites
- PMID: 32294508
- PMCID: PMC7317674
- DOI: 10.1016/j.mcn.2020.103492
Spatial control of membrane traffic in neuronal dendrites
Abstract
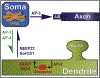
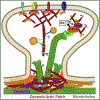
Neuronal dendrites are highly branched and specialized compartments with distinct structures and secretory organelles (e.g., spines, Golgi outposts), and a unique cytoskeletal organization that includes microtubules of mixed polarity. Dendritic membranes are enriched with proteins, which specialize in the formation and function of the post-synaptic membrane of the neuronal synapse. How these proteins partition preferentially in dendrites, and how they traffic in a manner that is spatiotemporally accurate and regulated by synaptic activity are long-standing questions of neuronal cell biology. Recent studies have shed new insights into the spatial control of dendritic membrane traffic, revealing new classes of proteins (e.g., septins) and cytoskeleton-based mechanisms with dendrite-specific functions. Here, we review these advances by revisiting the fundamental mechanisms that control membrane traffic at the levels of protein sorting and motor-driven transport on microtubules and actin filaments. Overall, dendrites possess unique mechanisms for the spatial control of membrane traffic, which might have specialized and co-evolved with their highly arborized morphology.
Keywords: Actin; Adaptor proteins; Axons; Dendrites; Kinesins; Membrane traffic; Microtubules; Myosins; Scaffolds; Septins; Sorting.
Copyright © 2020 Elsevier Inc. All rights reserved.
Figures



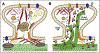
Similar articles
-
Polarity of Neuronal Membrane Traffic Requires Sorting of Kinesin Motor Cargo during Entry into Dendrites by a Microtubule-Associated Septin.Dev Cell. 2018 Jul 16;46(2):204-218.e7. doi: 10.1016/j.devcel.2018.06.013. Dev Cell. 2018. PMID: 30016622 Free PMC article.
-
Golgi Outposts Locally Regulate Microtubule Orientation in Neurons but Are Not Required for the Overall Polarity of the Dendritic Cytoskeleton.Genetics. 2020 Jun;215(2):435-447. doi: 10.1534/genetics.119.302979. Epub 2020 Apr 7. Genetics. 2020. PMID: 32265236 Free PMC article.
-
Which way to go? Cytoskeletal organization and polarized transport in neurons.Mol Cell Neurosci. 2011 Jan;46(1):9-20. doi: 10.1016/j.mcn.2010.08.015. Epub 2010 Sep 9. Mol Cell Neurosci. 2011. PMID: 20817096 Review.
-
Γ-tubulin controls neuronal microtubule polarity independently of Golgi outposts.Mol Biol Cell. 2014 Jul 1;25(13):2039-50. doi: 10.1091/mbc.E13-09-0515. Epub 2014 May 7. Mol Biol Cell. 2014. PMID: 24807906 Free PMC article.
-
Golgi Outposts Nucleate Microtubules in Cells with Specialized Shapes.Trends Cell Biol. 2020 Oct;30(10):792-804. doi: 10.1016/j.tcb.2020.07.004. Epub 2020 Aug 27. Trends Cell Biol. 2020. PMID: 32863092 Review.
Cited by
-
A novel labeling strategy reveals that myosin Va and myosin Vb bind the same dendritically polarized vesicle population.Traffic. 2020 Nov;21(11):689-701. doi: 10.1111/tra.12764. Traffic. 2020. PMID: 32959500 Free PMC article.
-
Cellular functions of actin- and microtubule-associated septins.Curr Biol. 2021 May 24;31(10):R651-R666. doi: 10.1016/j.cub.2021.03.064. Curr Biol. 2021. PMID: 34033796 Free PMC article. Review.
-
The dendritic engram.Front Behav Neurosci. 2023 Jul 27;17:1212139. doi: 10.3389/fnbeh.2023.1212139. eCollection 2023. Front Behav Neurosci. 2023. PMID: 37576932 Free PMC article.
-
NgCAM and VAMP2 reveal that direct delivery and dendritic degradation maintain axonal polarity.Mol Biol Cell. 2022 Jan 1;33(1):ar3. doi: 10.1091/mbc.E21-08-0425. Epub 2021 Nov 3. Mol Biol Cell. 2022. PMID: 34731031 Free PMC article.
-
The Subcortical-Allocortical- Neocortical continuum for the Emergence and Morphological Heterogeneity of Pyramidal Neurons in the Human Brain.Front Synaptic Neurosci. 2021 Mar 11;13:616607. doi: 10.3389/fnsyn.2021.616607. eCollection 2021. Front Synaptic Neurosci. 2021. PMID: 33776739 Free PMC article.
References
-
- Alberi S, Boda B, Steiner P, Nikonenko I, Hirling H, Muller D, 2005. The endosomal protein NEEP21 regulates AMPA receptor-mediated synaptic transmission and plasticity in the hippocampus. Mol Cell Neurosci 29, 313–319. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

