Pregnenolone Inhibits Osteoclast Differentiation and Protects Against Lipopolysaccharide-Induced Inflammatory Bone Destruction and Ovariectomy-Induced Bone Loss
- PMID: 32292342
- PMCID: PMC7135856
- DOI: 10.3389/fphar.2020.00360
Pregnenolone Inhibits Osteoclast Differentiation and Protects Against Lipopolysaccharide-Induced Inflammatory Bone Destruction and Ovariectomy-Induced Bone Loss
Abstract
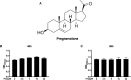
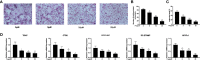
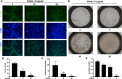
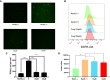
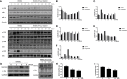
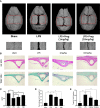
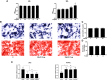
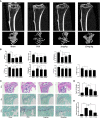
Osteolytic bone disease is characterized by excessive osteoclast bone resorption leading to increased skeletal fragility and fracture risk. Multinucleated osteoclasts formed through the fusion of mononuclear precursors are the principle cell capable of bone resorption. Pregnenolone (Preg) is the grand precursor of most if not all steroid hormones and have been suggested to be a novel anti-osteoporotic agent. However, the effects of Preg on osteoclast biology and function has yet to be shown. Here we examined the effect of Preg on receptor activator of nuclear factor kappa B ligand (RANKL)-induced osteoclast formation and bone resorption in vitro, and potential therapeutic application in inflammatory bone destruction and bone loss in vivo. Our in vitro cellular assays demonstrated that Preg can inhibit the formation of TRAP+ve osteoclast formation as well as mature osteoclast bone resorption in a dose-dependent manner. The expression of osteoclast marker genes CTSK, TRAP, DC-STAMP, ATP6V0d2, and NFATc1 were markedly attenuated. Biochemical analyses of RANKL-induced signaling pathways showed that Preg inhibited the early activation of extracellular regulated protein kinases (ERK) mitogen-activated protein kinase (MAPK) and nuclear factor-κB, which consequently impaired the downstream induction of c-Fos and NFATc1. Using reactive oxygen species (ROS) detection assays, we found that Preg exhibits anti-oxidant properties inhibiting the generation of intracellular ROS following RANKL stimulation. Consistent with these in vitro results, we confirmed that Preg protected mice against local Lipopolysaccharide (LPS)-induced inflammatory bone destruction in vivo by suppressing osteoclast formation. Furthermore, we did not find any observable effect of Preg on osteoblastogenesis and mineralization in vitro. Finally Preg was administered to ovariectomy (OVX)-induced bone loss and demonstrated that Preg prevented systemic OVX-induced osteoporosis. Collectively, our observations provide strong evidence for the use of Preg as anti-osteoclastogenic and anti-resorptive agent for the potential treatment of osteolytic bone conditions.
Keywords: ERK; LPS (lipopolysaccharide); OVX model; RANKL (receptor activator of nuclear factor kappa-B ligand); osteoclast (OCs).
Copyright © 2020 Sun, Zhang, Guo, Chen, Tao, Wang, Lin, Liu, Su and Qin.
Figures
Similar articles
-
Eleutherococcus sessiliflorus Inhibits Receptor Activator of Nuclear Factor Kappa-B Ligand (RANKL)-Induced Osteoclast Differentiation and Prevents Ovariectomy (OVX)-Induced Bone Loss.Molecules. 2021 Mar 26;26(7):1886. doi: 10.3390/molecules26071886. Molecules. 2021. PMID: 33810474 Free PMC article.
-
Gamabufotalin Inhibits Osteoclastgenesis and Counteracts Estrogen-Deficient Bone Loss in Mice by Suppressing RANKL-Induced NF-κB and ERK/MAPK Pathways.Front Pharmacol. 2021 Apr 23;12:629968. doi: 10.3389/fphar.2021.629968. eCollection 2021. Front Pharmacol. 2021. PMID: 33967763 Free PMC article.
-
Pseurotin A Inhibits Osteoclastogenesis and Prevents Ovariectomized-Induced Bone Loss by Suppressing Reactive Oxygen Species.Theranostics. 2019 Feb 28;9(6):1634-1650. doi: 10.7150/thno.30206. eCollection 2019. Theranostics. 2019. PMID: 31037128 Free PMC article.
-
Interleukin-27 prevents LPS-induced inflammatory osteolysis by inhibiting osteoclast formation and function.Am J Transl Res. 2019 Mar 15;11(3):1154-1169. eCollection 2019. Am J Transl Res. 2019. PMID: 30972153 Free PMC article. Review.
-
Caldecrin: A pancreas-derived hypocalcemic factor, regulates osteoclast formation and function.World J Biol Chem. 2015 Nov 26;6(4):358-65. doi: 10.4331/wjbc.v6.i4.358. World J Biol Chem. 2015. PMID: 26629319 Free PMC article. Review.
Cited by
-
The results of a unique dietary supplement (nutraceutical formulation) used to treat the symptoms of long-haul COVID.Front Nutr. 2022 Oct 25;9:1034169. doi: 10.3389/fnut.2022.1034169. eCollection 2022. Front Nutr. 2022. PMID: 36386945 Free PMC article.
-
Learning from Monocyte-Macrophage Fusion and Multinucleation: Potential Therapeutic Targets for Osteoporosis and Rheumatoid Arthritis.Int J Mol Sci. 2020 Aug 20;21(17):6001. doi: 10.3390/ijms21176001. Int J Mol Sci. 2020. PMID: 32825443 Free PMC article. Review.
-
Monotropein Protects against Inflammatory Bone Loss and Suppresses Osteoclast Formation and Bone Resorption by Inhibiting NFATc1 via NF-κB and Akt/GSK-3β Pathway.Nutrients. 2022 Sep 24;14(19):3978. doi: 10.3390/nu14193978. Nutrients. 2022. PMID: 36235631 Free PMC article.
-
Aminooxyacetic acid hemihydrochloride inhibits osteoclast differentiation and bone resorption by attenuating oxidative phosphorylation.Front Pharmacol. 2022 Sep 30;13:980678. doi: 10.3389/fphar.2022.980678. eCollection 2022. Front Pharmacol. 2022. PMID: 36249744 Free PMC article.
-
AEG-1 deletion promotes cartilage repair and modulates bone remodeling-related cytokines via TLR4/MyD88/NF-κB inhibition in ovariectomized rats with osteoporosis.Ann Transl Med. 2020 Oct;8(20):1298. doi: 10.21037/atm-20-5842. Ann Transl Med. 2020. PMID: 33209878 Free PMC article.
References
-
- Aliprantis A. O., Ueki Y., Sulyanto R., Park A., Sigrist K. S., Sharma S. M., et al. (2008). NFATc1 in mice represses osteoprotegerin during osteoclastogenesis and dissociates systemic osteopenia from inflammation in cherubism. J. Clin. Invest. 118 (11), 3775–3789. 10.1172/JCI35711 - DOI - PMC - PubMed
-
- Arnal J. F., Clamens S., Pechet C., Negre-Salvayre A., Allera C., Girolami J. P., et al. (1996). Ethinylestradiol does not enhance the expression of nitric oxide synthase in bovine endothelial cells but increases the release of bioactive nitric oxide by inhibiting superoxide anion production. Proc. Natl. Acad. Sci. U S A 93 (9), 4108–4113. 10.1073/pnas.93.9.4108 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

