Human Wharton's Jelly-Cellular Specificity, Stemness Potency, Animal Models, and Current Application in Human Clinical Trials
- PMID: 32290584
- PMCID: PMC7230974
- DOI: 10.3390/jcm9041102
Human Wharton's Jelly-Cellular Specificity, Stemness Potency, Animal Models, and Current Application in Human Clinical Trials
Abstract
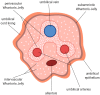
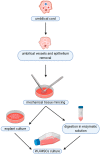
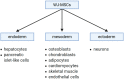
Stem cell therapies offer a great promise for regenerative and reconstructive medicine, due to their self-renewal and differentiation capacity. Although embryonic stem cells are pluripotent, their utilization involves embryo destruction and is ethically controversial. Therefore, adult tissues that have emerged as an alternative source of stem cells and perinatal tissues, such as the umbilical cord, appear to be particularly attractive. Wharton's jelly, a gelatinous connective tissue contained in the umbilical cord, is abundant in mesenchymal stem cells (MSCs) that express CD105, CD73, CD90, Oct-4, Sox-2, and Nanog among others, and have the ability to differentiate into osteogenic, adipogenic, chondrogenic, and other lineages. Moreover, Wharton's jelly-derived MSCs (WJ-MSCs) do not express MHC-II and exhibit immunomodulatory properties, which makes them a good alternative for allogeneic and xenogeneic transplantations in cellular therapies. Therefore, umbilical cord, especially Wharton's jelly, is a promising source of mesenchymal stem cells.
Keywords: Wharton’s jelly; stem cells; umbilical cord.
Conflict of interest statement
The authors declare they have no conflict of interest.
Figures
Similar articles
-
The effect of fibroblast growth factor on distinct differentiation potential of cord blood-derived unrestricted somatic stem cells and Wharton's jelly-derived mesenchymal stem/stromal cells.Cytotherapy. 2015 Dec;17(12):1723-31. doi: 10.1016/j.jcyt.2015.09.007. Cytotherapy. 2015. PMID: 26589753
-
Comparative analysis of human Wharton's jelly mesenchymal stem cells derived from different parts of the same umbilical cord.Cell Tissue Res. 2018 Apr;372(1):51-65. doi: 10.1007/s00441-017-2699-4. Epub 2017 Dec 4. Cell Tissue Res. 2018. PMID: 29204746 Free PMC article.
-
Pluripotent gene expression in mesenchymal stem cells from human umbilical cord Wharton's jelly and their differentiation potential to neural-like cells.J Med Assoc Thai. 2013 Sep;96(9):1208-17. J Med Assoc Thai. 2013. PMID: 24163998
-
New emerging potentials for human Wharton's jelly mesenchymal stem cells: immunological features and hepatocyte-like differentiative capacity.Stem Cells Dev. 2010 Apr;19(4):423-38. doi: 10.1089/scd.2009.0299. Stem Cells Dev. 2010. PMID: 19958166 Review.
-
Wharton's jelly-derived stromal cells and their cell therapy applications in allogeneic haematopoietic stem cell transplantation.J Cell Mol Med. 2022 Mar;26(5):1339-1350. doi: 10.1111/jcmm.17105. Epub 2022 Jan 28. J Cell Mol Med. 2022. PMID: 35088933 Free PMC article. Review.
Cited by
-
Placental Tissues as Biomaterials in Regenerative Medicine.Biomed Res Int. 2022 Apr 21;2022:6751456. doi: 10.1155/2022/6751456. eCollection 2022. Biomed Res Int. 2022. PMID: 35496035 Free PMC article. Review.
-
Regulating the fate of stem cells for regenerating the intervertebral disc degeneration.World J Stem Cells. 2021 Dec 26;13(12):1881-1904. doi: 10.4252/wjsc.v13.i12.1881. World J Stem Cells. 2021. PMID: 35069988 Free PMC article. Review.
-
Mesenchymal stem cells express epidermal markers in an in vitro reconstructed human skin model.Front Cell Dev Biol. 2023 Jan 12;10:1012637. doi: 10.3389/fcell.2022.1012637. eCollection 2022. Front Cell Dev Biol. 2023. PMID: 36712971 Free PMC article.
-
The order of green and red LEDs irradiation affects the neural differentiation of human umbilical cord matrix-derived mesenchymal cells.Sci Rep. 2024 Sep 10;14(1):21107. doi: 10.1038/s41598-024-72099-3. Sci Rep. 2024. PMID: 39256554 Free PMC article.
-
Application of Wharton jelly-derived mesenchymal stem cells in patients with pulmonary fibrosis.Stem Cell Res Ther. 2022 Feb 15;13(1):71. doi: 10.1186/s13287-022-02746-x. Stem Cell Res Ther. 2022. PMID: 35168663 Free PMC article. Review.
References
-
- Doğan A. Embryonic stem cells in development and regenerative medicine. Adv. Exp. Med. Biol. 2018;1079:1–15. - PubMed
-
- Gruber H.E., Deepe R., Hoelscher G.L., Ingram J.A., Norton H.J., Scannell B., Loeffler B.J., Zinchenko N., Hanley E.N., Tapp H. Human adipose-derived mesenchymal stem cells: Direction to a phenotype sharing similarities with the disc, gene expression profiling, and coculture with human annulus cells. Tissue Eng. Part A. 2010;16:2843–2860. doi: 10.1089/ten.tea.2009.0709. - DOI - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

