Visible Light as an Antimicrobial Strategy for Inactivation of Pseudomonas fluorescens and Staphylococcus epidermidis Biofilms
- PMID: 32290162
- PMCID: PMC7235755
- DOI: 10.3390/antibiotics9040171
Visible Light as an Antimicrobial Strategy for Inactivation of Pseudomonas fluorescens and Staphylococcus epidermidis Biofilms
Abstract
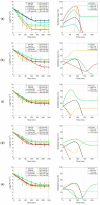
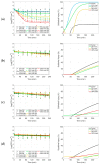
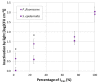
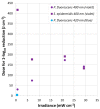
The increase of antimicrobial resistance is challenging the scientific community to find solutions to eradicate bacteria, specifically biofilms. Light-Emitting Diodes (LED) represent an alternative way to tackle this problem in the presence of endogenous or exogenous photosensitizers. This work adds to a growing body of research on photodynamic inactivation using visible light against biofilms. Violet (400 nm), blue (420 nm), green (570 nm), yellow (584 nm) and red (698 nm) LEDs were used against Pseudomonas fluorescens and Staphylococcus epidermidis. Biofilms, grown on a polystyrene surface, were irradiated for 4 h. Different irradiance levels were investigated (2.5%, 25%, 50% and 100% of the maximum irradiance). Surviving cells were quantified and the inactivation kinetic parameters were estimated. Violet light could successfully inactivate P. fluorescens and S. epidermidis (up to 6.80 and 3.69 log10 reduction, respectively), while blue light was effective only against P. fluorescens (100% of maximum irradiance). Green, yellow and red irradiation neither increased nor reduced the biofilm cell density. This is the first research to test five different wavelengths (each with three intensities) in the visible spectrum against Gram-positive and Gram-negative biofilms. It provides a detailed study of the potential of visible light against biofilms of a different Gram-nature.
Keywords: Biofilm; Pseudomonas fluorescens; Staphylococcus epidermidis; antimicrobial inactivation; disinfection; inactivation kinetics; light-emitting diode; photodynamic inactivation; polystyrene surface; visible light.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
The microbicidal potential of visible blue light in clinical medicine and public health.Front Med (Lausanne). 2022 Jul 22;9:905606. doi: 10.3389/fmed.2022.905606. eCollection 2022. Front Med (Lausanne). 2022. PMID: 35935800 Free PMC article. Review.
-
Ultra-efficient antimicrobial photodynamic inactivation system based on blue light and octyl gallate for ablation of planktonic bacteria and biofilms of Pseudomonas fluorescens.Food Chem. 2022 Apr 16;374:131585. doi: 10.1016/j.foodchem.2021.131585. Epub 2021 Nov 11. Food Chem. 2022. PMID: 34802804
-
Violet-Blue Light Arrays at 405 Nanometers Exert Enhanced Antimicrobial Activity for Photodisinfection of Monomicrobial Nosocomial Biofilms.Appl Environ Microbiol. 2019 Oct 16;85(21):e01346-19. doi: 10.1128/AEM.01346-19. Print 2019 Nov 1. Appl Environ Microbiol. 2019. PMID: 31444205 Free PMC article.
-
Characterization of Blue Light Treatment for Infected Wounds: Antibacterial Efficacy of 420, 455, and 480 nm Light-Emitting Diode Arrays Against Common Skin Pathogens Versus Blue Light-Induced Skin Cell Toxicity.Photobiomodul Photomed Laser Surg. 2021 May;39(5):339-348. doi: 10.1089/photob.2020.4932. Photobiomodul Photomed Laser Surg. 2021. PMID: 33961502
-
Blue light for infectious diseases: Propionibacterium acnes, Helicobacter pylori, and beyond?Drug Resist Updat. 2012 Aug;15(4):223-36. doi: 10.1016/j.drup.2012.07.001. Epub 2012 Jul 28. Drug Resist Updat. 2012. PMID: 22846406 Free PMC article. Review.
Cited by
-
The Microbial Metagenome of Eluates Obtained From the Surface of Broccoli Heads Subjected to Different Light Treatments.Front Microbiol. 2022 Apr 13;13:820419. doi: 10.3389/fmicb.2022.820419. eCollection 2022. Front Microbiol. 2022. PMID: 35495709 Free PMC article.
-
New Trends in Photodynamic Inactivation (PDI) Combating Biofilms in the Food Industry-A Review.Foods. 2021 Oct 26;10(11):2587. doi: 10.3390/foods10112587. Foods. 2021. PMID: 34828868 Free PMC article. Review.
-
Antimicrobial blue light: A 'Magic Bullet' for the 21st century and beyond?Adv Drug Deliv Rev. 2022 Jan;180:114057. doi: 10.1016/j.addr.2021.114057. Epub 2021 Nov 18. Adv Drug Deliv Rev. 2022. PMID: 34800566 Free PMC article. Review.
-
The microbicidal potential of visible blue light in clinical medicine and public health.Front Med (Lausanne). 2022 Jul 22;9:905606. doi: 10.3389/fmed.2022.905606. eCollection 2022. Front Med (Lausanne). 2022. PMID: 35935800 Free PMC article. Review.
-
The Composites of PCL and Tetranuclear Titanium(IV)-oxo Complexes as Materials Exhibiting the Photocatalytic and the Antimicrobial Activity.Int J Mol Sci. 2021 Jun 29;22(13):7021. doi: 10.3390/ijms22137021. Int J Mol Sci. 2021. PMID: 34209889 Free PMC article.
References
-
- Seviour T., Derlon N., Du M., Flemming H.-C., Girbal-Neuhauser E., Horn H., Kjelleberg S., van Loosdrecht M.C.M., Lotti T., Malpei M.F., et al. Extracellular polymeric substances of biofilms: Suffering from an identity crisis. Water Res. 2019;151:1–7. doi: 10.1016/j.watres.2018.11.020. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

