Virus-Host Interactions Between Nonsecretors and Human Norovirus
- PMID: 32289501
- PMCID: PMC7301201
- DOI: 10.1016/j.jcmgh.2020.03.006
Virus-Host Interactions Between Nonsecretors and Human Norovirus
Abstract
Background & aims: Human norovirus infection is the leading cause of acute gastroenteritis. Genetic polymorphisms, mediated by the FUT2 gene (secretor enzyme), define strain susceptibility. Secretors express a diverse set of fucosylated histoblood group antigen carbohydrates (HBGA) on mucosal cells; nonsecretors (FUT2-/-) express a limited array of HBGAs. Thus, nonsecretors have less diverse norovirus strain infections, including resistance to the epidemiologically dominant GII.4 strains. Because future human norovirus vaccines will comprise GII.4 antigen and because secretor phenotype impacts GII.4 infection and immunity, nonsecretors may mimic young children immunologically in response to GII.4 vaccination, providing a needed model to study cross-protection in the context of limited pre-exposure.
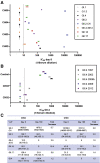
Methods: By using specimens collected from the first characterized nonsecretor cohort naturally infected with GII.2 human norovirus, we evaluated the breadth of serologic immunity by surrogate neutralization assays, and cellular activation and cytokine production by flow cytometry.
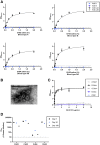
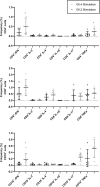
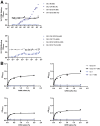
Results: GII.2 infection resulted in broad antibody and cellular immunity activation that persisted for at least 30 days for T cells, monocytes, and dendritic cells, and for 180 days for blocking antibody. Multiple cellular lineages expressing interferon-γ and tumor necrosis factor-α dominated the response. Both T-cell and B-cell responses were cross-reactive with other GII strains, but not GI strains. To promote entry mechanisms, inclusion of bile acids was essential for GII.2 binding to nonsecretor HBGAs.
Conclusions: These data support development of within-genogroup, cross-reactive antibody and T-cell immunity, key outcomes that may provide the foundation for eliciting broad immune responses after GII.4 vaccination in individuals with limited GII.4 immunity, including young children.
Keywords: Bile; Blockade Antibody; Cellular Immunity; Neutralizing Antibody; Receptor Binding.
Copyright © 2020 The Authors. Published by Elsevier Inc. All rights reserved.
Figures









Comment in
-
Towards a Comprehensive Understanding of Human Norovirus Immunity.Cell Mol Gastroenterol Hepatol. 2020;10(2):422-423. doi: 10.1016/j.jcmgh.2020.04.019. Epub 2020 May 29. Cell Mol Gastroenterol Hepatol. 2020. PMID: 32479756 Free PMC article. No abstract available.
Similar articles
-
Genetic Manipulation of Human Intestinal Enteroids Demonstrates the Necessity of a Functional Fucosyltransferase 2 Gene for Secretor-Dependent Human Norovirus Infection.mBio. 2020 Mar 17;11(2):e00251-20. doi: 10.1128/mBio.00251-20. mBio. 2020. PMID: 32184242 Free PMC article.
-
Broad blockade antibody responses in human volunteers after immunization with a multivalent norovirus VLP candidate vaccine: immunological analyses from a phase I clinical trial.PLoS Med. 2015 Mar 24;12(3):e1001807. doi: 10.1371/journal.pmed.1001807. eCollection 2015 Mar. PLoS Med. 2015. PMID: 25803642 Free PMC article. Clinical Trial.
-
Association between norovirus and rotavirus infection and histo-blood group antigen types in Vietnamese children.J Clin Microbiol. 2014 May;52(5):1366-74. doi: 10.1128/JCM.02927-13. Epub 2014 Feb 12. J Clin Microbiol. 2014. PMID: 24523471 Free PMC article.
-
Mendelian resistance to human norovirus infections.Semin Immunol. 2006 Dec;18(6):375-86. doi: 10.1016/j.smim.2006.07.009. Epub 2006 Sep 14. Semin Immunol. 2006. PMID: 16973373 Free PMC article. Review.
-
Norovirus and histo-blood group antigens.Jpn J Infect Dis. 2011;64(2):95-103. Jpn J Infect Dis. 2011. PMID: 21519121 Review.
Cited by
-
Immune Imprinting Drives Human Norovirus Potential for Global Spread.mBio. 2022 Oct 26;13(5):e0186122. doi: 10.1128/mbio.01861-22. Epub 2022 Sep 14. mBio. 2022. PMID: 36102514 Free PMC article.
-
Roles of bile acids in enteric virus replication.Anim Dis. 2021;1(1):2. doi: 10.1186/s44149-021-00003-x. Epub 2021 Apr 23. Anim Dis. 2021. PMID: 34778876 Free PMC article. Review.
-
Biological and immunological characterization of major capsid protein VP1 from distinct GII.2 norovirus clusters.Sci Rep. 2024 Sep 9;14(1):21035. doi: 10.1038/s41598-024-72062-2. Sci Rep. 2024. PMID: 39251865 Free PMC article.
-
Serological Humoral Immunity Following Natural Infection of Children with High Burden Gastrointestinal Viruses.Viruses. 2021 Oct 9;13(10):2033. doi: 10.3390/v13102033. Viruses. 2021. PMID: 34696463 Free PMC article. Review.
-
Glycan Recognition in Human Norovirus Infections.Viruses. 2021 Oct 14;13(10):2066. doi: 10.3390/v13102066. Viruses. 2021. PMID: 34696500 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

