Collagen-binding IL-12 enhances tumour inflammation and drives the complete remission of established immunologically cold mouse tumours
- PMID: 32284554
- PMCID: PMC11095084
- DOI: 10.1038/s41551-020-0549-2
Collagen-binding IL-12 enhances tumour inflammation and drives the complete remission of established immunologically cold mouse tumours
Abstract
Checkpoint-inhibitor (CPI) immunotherapy has achieved remarkable clinical success, yet its efficacy in 'immunologically cold' tumours has been modest. Interleukin-12 (IL-12) is a powerful cytokine that activates the innate and adaptive arms of the immune system; however, the administration of IL-12 has been associated with immune-related adverse events. Here we show that, after intravenous administration of a collagen-binding domain fused to IL-12 (CBD-IL-12) in mice bearing aggressive mouse tumours, CBD-IL-12 accumulates in the tumour stroma due to exposed collagen in the disordered tumour vasculature. In comparison with the administration of unmodified IL-12, CBD-IL-12 induced sustained intratumoural levels of interferon-γ, substantially reduced its systemic levels as well as organ damage and provided superior anticancer efficacy, eliciting complete regression of CPI-unresponsive breast tumours. Furthermore, CBD-IL-12 potently synergized with CPI to eradicate large established melanomas, induced antigen-specific immunological memory and controlled tumour growth in a genetically engineered mouse model of melanoma. CBD-IL-12 may potentiate CPI immunotherapy for immunologically cold tumours.
Figures
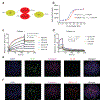


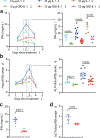

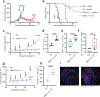
Comment in
-
Collagen-binding IL-12 inflames 'cold' tumours.Nat Biomed Eng. 2020 May;4(5):483-484. doi: 10.1038/s41551-020-0560-7. Nat Biomed Eng. 2020. PMID: 32409704 No abstract available.
Similar articles
-
Targeted antibody and cytokine cancer immunotherapies through collagen affinity.Sci Transl Med. 2019 Apr 10;11(487):eaau3259. doi: 10.1126/scitranslmed.aau3259. Sci Transl Med. 2019. PMID: 30971453 Free PMC article.
-
Masking the immunotoxicity of interleukin-12 by fusing it with a domain of its receptor via a tumour-protease-cleavable linker.Nat Biomed Eng. 2022 Jul;6(7):819-829. doi: 10.1038/s41551-022-00888-0. Epub 2022 May 9. Nat Biomed Eng. 2022. PMID: 35534574 Free PMC article.
-
An IL-12-Based Nanocytokine Safely Potentiates Anticancer Immunity through Spatiotemporal Control of Inflammation to Eradicate Advanced Cold Tumors.Adv Sci (Weinh). 2023 Apr;10(10):e2205139. doi: 10.1002/advs.202205139. Epub 2023 Feb 5. Adv Sci (Weinh). 2023. PMID: 36739605 Free PMC article.
-
Intratumoural immunotherapies in oncology.Eur J Cancer. 2020 Mar;127:1-11. doi: 10.1016/j.ejca.2019.12.007. Epub 2020 Jan 18. Eur J Cancer. 2020. PMID: 31962197 Review.
-
Inflammasomes: Emerging Central Players in Cancer Immunology and Immunotherapy.Front Immunol. 2018 Dec 20;9:3028. doi: 10.3389/fimmu.2018.03028. eCollection 2018. Front Immunol. 2018. PMID: 30631327 Free PMC article. Review.
Cited by
-
Exploiting protein domain modularity to enable synthetic control of engineered cells.Curr Opin Biomed Eng. 2024 Sep;31:100550. doi: 10.1016/j.cobme.2024.100550. Epub 2024 Jul 2. Curr Opin Biomed Eng. 2024. PMID: 39430298
-
Interleukins in cancer: from biology to therapy.Nat Rev Cancer. 2021 Aug;21(8):481-499. doi: 10.1038/s41568-021-00363-z. Epub 2021 Jun 3. Nat Rev Cancer. 2021. PMID: 34083781 Free PMC article. Review.
-
High-Affinity Superantigen-Based Trifunctional Immune Cell Engager Synergizes NK and T Cell Activation for Tumor Suppression.Adv Sci (Weinh). 2024 Sep;11(33):e2310204. doi: 10.1002/advs.202310204. Epub 2024 Jun 27. Adv Sci (Weinh). 2024. PMID: 38937984 Free PMC article.
-
Emerging principles of cytokine pharmacology and therapeutics.Nat Rev Drug Discov. 2023 Jan;22(1):21-37. doi: 10.1038/s41573-022-00557-6. Epub 2022 Sep 21. Nat Rev Drug Discov. 2023. PMID: 36131080 Free PMC article. Review.
-
Nanocarriers for cancer nano-immunotherapy.Drug Deliv Transl Res. 2023 Jul;13(7):1936-1954. doi: 10.1007/s13346-022-01241-3. Epub 2022 Oct 3. Drug Deliv Transl Res. 2023. PMID: 36190661 Free PMC article. Review.
References
-
- Sharma P. & Allison JP The future of immune checkpoint therapy. Science 348, 56–61 (2015). - PubMed
-
- Langrish CL et al. IL-12 and IL-23: master regulators of innate and adaptive immunity. Immunol Rev 202, 96–105 (2004). - PubMed
-
- McHeyzer-Williams MG et al. Antigen-specific immunity. Immunologic Research 22, 223–236 (2000). - PubMed
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources

