The cGAS/STING/TBK1/IRF3 innate immunity pathway maintains chromosomal stability through regulation of p21 levels
- PMID: 32284536
- PMCID: PMC7210884
- DOI: 10.1038/s12276-020-0416-y
The cGAS/STING/TBK1/IRF3 innate immunity pathway maintains chromosomal stability through regulation of p21 levels
Abstract
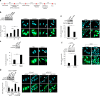
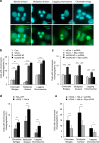
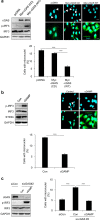
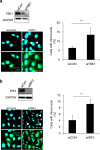
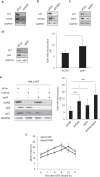
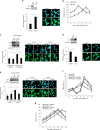
Chromosomal instability (CIN) in cancer cells has been reported to activate the cGAS-STING innate immunity pathway via micronuclei formation, thus affecting tumor immunity and tumor progression. However, adverse effects of the cGAS/STING pathway as they relate to CIN have not yet been investigated. We addressed this issue using knockdown and add-back approaches to analyze each component of the cGAS/STING/TBK1/IRF3 pathway, and we monitored the extent of CIN by measuring micronuclei formation after release from nocodazole-induced mitotic arrest. Interestingly, knockdown of cGAS (cyclic GMP-AMP synthase) along with induction of mitotic arrest in HeLa and U2OS cancer cells clearly resulted in increased micronuclei formation and chromosome missegregation. Knockdown of STING (stimulator of interferon genes), TBK1 (TANK-binding kinase-1), or IRF3 (interferon regulatory factor-3) also resulted in increased micronuclei formation. Moreover, transfection with cGAMP, the product of cGAS enzymatic activity, as well as add-back of cGAS WT (but not catalytic-dead mutant cGAS), or WT or constitutively active STING (but not an inactive STING mutant) rescued the micronuclei phenotype, demonstrating that all components of the cGAS/STING/TBK1/IRF3 pathway play a role in preventing CIN. Moreover, p21 levels were decreased in cGAS-, STING-, TBK1-, and IRF3-knockdown cells, which was accompanied by the precocious G2/M transition of cells and the enhanced micronuclei phenotype. Overexpression of p21 or inhibition of CDK1 in cGAS-depleted cells reduced micronuclei formation and abrogated the precocious G2/M transition, indicating that the decrease in p21 and the subsequent precocious G2/M transition is the main mechanism underlying the induction of CIN through disruption of cGAS/STING signaling.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures

Similar articles
-
Micronuclei induced by radiation, replication stress, or chromosome segregation errors do not activate cGAS-STING.Mol Cell. 2024 Jun 6;84(11):2203-2213.e5. doi: 10.1016/j.molcel.2024.04.017. Epub 2024 May 14. Mol Cell. 2024. PMID: 38749421
-
African Swine Fever Virus Armenia/07 Virulent Strain Controls Interferon Beta Production through the cGAS-STING Pathway.J Virol. 2019 May 29;93(12):e02298-18. doi: 10.1128/JVI.02298-18. Print 2019 Jun 15. J Virol. 2019. PMID: 30918080 Free PMC article.
-
DNA damage-triggered activation of cGAS-STING pathway induces apoptosis in human keratinocyte HaCaT cells.Mol Immunol. 2021 Mar;131:180-190. doi: 10.1016/j.molimm.2020.12.037. Epub 2021 Jan 8. Mol Immunol. 2021. PMID: 33423764
-
The molecular mechanism of dsDNA sensing through the cGAS-STING pathway.Adv Immunol. 2024;162:1-21. doi: 10.1016/bs.ai.2024.02.003. Epub 2024 Mar 2. Adv Immunol. 2024. PMID: 38866436 Review.
-
The mechanism of double-stranded DNA sensing through the cGAS-STING pathway.Cytokine Growth Factor Rev. 2014 Dec;25(6):641-8. doi: 10.1016/j.cytogfr.2014.06.006. Epub 2014 Jun 22. Cytokine Growth Factor Rev. 2014. PMID: 25007740 Free PMC article. Review.
Cited by
-
STING Contributes to Cancer-Induced Bone Pain by Promoting M1 Polarization of Microglia in the Medial Prefrontal Cortex.Cancers (Basel). 2022 Oct 22;14(21):5188. doi: 10.3390/cancers14215188. Cancers (Basel). 2022. PMID: 36358605 Free PMC article.
-
Sensing Stemness.Curr Stem Cell Rep. 2021;7(4):219-228. doi: 10.1007/s40778-021-00201-w. Epub 2021 Oct 6. Curr Stem Cell Rep. 2021. PMID: 34868827 Free PMC article. Review.
-
Genotoxic stress in constitutive trisomies induces autophagy and the innate immune response via the cGAS-STING pathway.Commun Biol. 2021 Jul 2;4(1):831. doi: 10.1038/s42003-021-02278-9. Commun Biol. 2021. PMID: 34215848 Free PMC article.
-
The role of DNA damage response in amyotrophic lateral sclerosis.Essays Biochem. 2020 Oct 26;64(5):847-861. doi: 10.1042/EBC20200002. Essays Biochem. 2020. PMID: 33078197 Free PMC article. Review.
-
STING agonists as promising vaccine adjuvants to boost immunogenicity against SARS-related coronavirus derived infection: possible role of autophagy.Cell Commun Signal. 2024 Jun 3;22(1):305. doi: 10.1186/s12964-024-01680-0. Cell Commun Signal. 2024. PMID: 38831299 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

