ER stress activates immunosuppressive network: implications for aging and Alzheimer's disease
- PMID: 32279085
- PMCID: PMC7220864
- DOI: 10.1007/s00109-020-01904-z
ER stress activates immunosuppressive network: implications for aging and Alzheimer's disease
Abstract
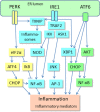
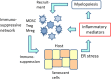
The endoplasmic reticulum (ER) contains stress sensors which recognize the accumulation of unfolded proteins within the lumen of ER, and subsequently these transducers stimulate the unfolded protein response (UPR). The ER sensors include the IRE1, PERK, and ATF6 transducers which activate the UPR in an attempt to restore the quality of protein folding and thus maintain cellular homeostasis. If there is excessive stress, UPR signaling generates alarmins, e.g., chemokines and cytokines, which activate not only tissue-resident immune cells but also recruit myeloid and lymphoid cells into the affected tissues. ER stress is a crucial inducer of inflammation in many pathological conditions. A chronic low-grade inflammation and cellular senescence have been associated with the aging process and many age-related diseases, such as Alzheimer's disease. Currently, it is known that immune cells can exhibit great plasticity, i.e., they are able to display both pro-inflammatory and anti-inflammatory phenotypes in a context-dependent manner. The microenvironment encountered in chronic inflammatory conditions triggers a compensatory immunosuppression which defends tissues from excessive inflammation. Recent studies have revealed that chronic ER stress augments the suppressive phenotypes of immune cells, e.g., in tumors and other inflammatory disorders. The activation of immunosuppressive network, including myeloid-derived suppressor cells (MDSC) and regulatory T cells (Treg), has been involved in the aging process and Alzheimer's disease. We will examine in detail whether the ER stress-related changes found in aging tissues and Alzheimer's disease are associated with the activation of immunosuppressive network, as has been observed in tumors and many chronic inflammatory diseases.
Keywords: Ageing; Immunometabolism; Immunosenescence; Immunosuppression; Inflammaging; Neurodegeneration.
Conflict of interest statement
The authors state that there are no personal or institutional conflicts of interest.
Figures
Similar articles
-
Activation of immunosuppressive network in the aging process.Ageing Res Rev. 2020 Jan;57:100998. doi: 10.1016/j.arr.2019.100998. Epub 2019 Dec 12. Ageing Res Rev. 2020. PMID: 31838128 Review.
-
Increased immunosuppression impairs tissue homeostasis with aging and age-related diseases.J Mol Med (Berl). 2021 Jan;99(1):1-20. doi: 10.1007/s00109-020-01988-7. Epub 2020 Oct 6. J Mol Med (Berl). 2021. PMID: 33025106 Free PMC article. Review.
-
ER stress in Alzheimer's disease: a novel neuronal trigger for inflammation and Alzheimer's pathology.J Neuroinflammation. 2009 Dec 26;6:41. doi: 10.1186/1742-2094-6-41. J Neuroinflammation. 2009. PMID: 20035627 Free PMC article. Review.
-
Exploring ER stress response in cellular aging and neuroinflammation in Alzheimer's disease.Ageing Res Rev. 2021 Sep;70:101417. doi: 10.1016/j.arr.2021.101417. Epub 2021 Jul 31. Ageing Res Rev. 2021. PMID: 34339860 Review.
-
Crosstalk between endoplasmic reticulum stress and brain inflammation in Alzheimer's disease.Neuropharmacology. 2018 Jul 1;136(Pt B):350-360. doi: 10.1016/j.neuropharm.2017.11.016. Epub 2017 Nov 10. Neuropharmacology. 2018. PMID: 29129774 Review.
Cited by
-
Curcumin Reduces Cognitive Deficits by Inhibiting Neuroinflammation through the Endoplasmic Reticulum Stress Pathway in Apolipoprotein E4 Transgenic Mice.ACS Omega. 2021 Mar 2;6(10):6654-6662. doi: 10.1021/acsomega.0c04810. eCollection 2021 Mar 16. ACS Omega. 2021. PMID: 33748578 Free PMC article.
-
Gene body DNA hydroxymethylation restricts the magnitude of transcriptional changes during aging.Nat Commun. 2024 Jul 28;15(1):6357. doi: 10.1038/s41467-024-50725-y. Nat Commun. 2024. PMID: 39069555 Free PMC article.
-
The Uncovered Function of the Drosophila GBA1a-Encoded Protein.Cells. 2021 Mar 12;10(3):630. doi: 10.3390/cells10030630. Cells. 2021. PMID: 33809074 Free PMC article.
-
Isoquercitrin Induces Endoplasmic Reticulum Stress and Immunogenic Cell Death in Gastric Cancer Cells.Biochem Genet. 2023 Jun;61(3):1128-1142. doi: 10.1007/s10528-022-10309-1. Epub 2022 Dec 8. Biochem Genet. 2023. PMID: 36480095
-
Transcriptome and Literature Mining Highlight the Differential Expression of ERLIN1 in Immune Cells during Sepsis.Biology (Basel). 2021 Aug 5;10(8):755. doi: 10.3390/biology10080755. Biology (Basel). 2021. PMID: 34439987 Free PMC article.
References
-
- Ron D, Walter P. Signal integration in the endoplasmic reticulum unfolded protein response. Nat Rev Mol Cell Biol. 2007;8:519–529. - PubMed
-
- Xiang C, Wang Y, Zhang H, Han F. The role of endoplasmic reticulum stress in neurodegenerative disease. Apoptosis. 2017;22:1–26. - PubMed
-
- Amodio G, Cichy J, Conde P, Matteoli G, Moreau A, Ochando J, Oral BH, Pekarova M, Ryan EJ, Roth J, Sohrabi Y, Cuturi MC, Gregori S. Role of myeloid regulatory cells (MRCs) in maintaining tissue homeostasis and promoting tolerance in autoimmunity, inflammatory disease and transplantation. Cancer Immunol Immunother. 2019;68:661–672. - PMC - PubMed
-
- Umansky V, Adema GJ, Baran J, Brandau S, Van Ginderachter JA, Hu X, Jablonska J, Mojsilovic S, Papadaki HA, Pico de Coana Y, Santegoets KCM, Santibanez JF, Serre K, Si Y, Sieminska I, Velegraki M, Fridlender ZG. Interactions among myeloid regulatory cells in cancer. Cancer Immunol Immunother. 2019;68:645–660. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

