Long noncoding RNA MEG3 decreases the growth of head and neck squamous cell carcinoma by regulating the expression of miR-421 and E-cadherin
- PMID: 32277605
- PMCID: PMC7286453
- DOI: 10.1002/cam4.3002
Long noncoding RNA MEG3 decreases the growth of head and neck squamous cell carcinoma by regulating the expression of miR-421 and E-cadherin
Abstract
Background: Maternally expressed 3 (MEG3), a long chain noncoding RNA (lncRNA), has verified its function as a suppressor in several kinds of cancers. However, the downstream mechanism of MEG3 in regulating the molecular mechanism of epithelial-mesenchymal transformation (EMT) in head and neck squamous cell carcinoma (HNSCC) progression demands further investigation.
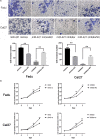
Methods: Quantitative real-time polymerase chain reaction (qRT-PCR) was used to determine the expression level of MEG3 in HNSCC and adjacent normal tissues of 51 cases. Luciferase report assay was used to detect the correlation between miR-421 and MEG3, and miR-421 and E-cadherin in HNSCC cell lines. Cell invasion and proliferation capacity were assessed through transwell and CCK8 assays. Scratch wound assay was used to assess cell migration capacity.
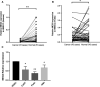
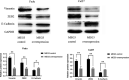
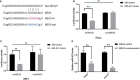
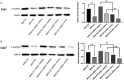
Results: Firstly, this study demonstrated that the expression of MEG3 was significantly downregulated in HNSCC compared to adjacent normal tissues. Overexpressed MEG3 inhibited cell proliferation, migration, and invasion in vitro. Secondly, MEG3 upregulated the expression of E-cadherin, which was instead downregulated by miR-421. MiR-421 was negatively regulated by MEG3 in HNSCC. Therefore, MEG3 regulated EMT by sponging miR-421 targeting E-cadherin in HNSCC.
Conclusions: This study indicated that the MEG3-miR-421-E-cadherin axis could be a new therapeutic target for HNSCC.
Keywords: epithelial-mesenchymal transition; head and neck squamous cell carcinoma; maternally expressed 3; miR-421.
© 2020 The Authors. Cancer Medicine published by John Wiley & Sons Ltd.
Conflict of interest statement
None.
Figures


Similar articles
-
LncRNA MEG3 inhibits cell epithelial-mesenchymal transition by sponging miR-421 targeting E-cadherin in breast cancer.Biomed Pharmacother. 2017 Jul;91:312-319. doi: 10.1016/j.biopha.2017.04.085. Epub 2017 May 2. Biomed Pharmacother. 2017. PMID: 28463794
-
Low expression of lncRNA MEG3 promotes the progression of oral squamous cell carcinoma by targeting miR-21.Eur Rev Med Pharmacol Sci. 2018 Dec;22(23):8315-8323. doi: 10.26355/eurrev_201812_16529. Eur Rev Med Pharmacol Sci. 2018. PMID: 30556872
-
Long Noncoding RNA LINC00460 Promotes Cell Progression by Sponging miR-4443 in Head and Neck Squamous Cell Carcinoma.Cell Transplant. 2020 Jan-Dec;29:963689720927405. doi: 10.1177/0963689720927405. Cell Transplant. 2020. PMID: 32478564 Free PMC article.
-
Long noncoding RNAs in head and neck squamous cell carcinoma: biological functions and mechanisms.Mol Biol Rep. 2020 Oct;47(10):8075-8090. doi: 10.1007/s11033-020-05777-w. Epub 2020 Sep 10. Mol Biol Rep. 2020. PMID: 32914266 Review.
-
Long non-coding RNAs orchestrate various molecular and cellular processes by modulating epithelial-mesenchymal transition in head and neck squamous cell carcinoma.Biochim Biophys Acta Mol Basis Dis. 2021 Nov 1;1867(11):166240. doi: 10.1016/j.bbadis.2021.166240. Epub 2021 Aug 5. Biochim Biophys Acta Mol Basis Dis. 2021. PMID: 34363933 Review.
Cited by
-
Treatment dependent impact of plasma-derived exosomes from head and neck cancer patients on the epithelial-to-mesenchymal transition.Front Oncol. 2023 Jan 4;12:1043199. doi: 10.3389/fonc.2022.1043199. eCollection 2022. Front Oncol. 2023. PMID: 36686733 Free PMC article.
-
A Cell Component-Related Prognostic Signature for Head and Neck Squamous Cell Carcinoma Based on the Tumor Microenvironment.Int J Genomics. 2022 Jun 25;2022:6022869. doi: 10.1155/2022/6022869. eCollection 2022. Int J Genomics. 2022. PMID: 35795712 Free PMC article.
-
A deep learning method for classification of HNSCC and HPV patients using single-cell transcriptomics.Front Mol Biosci. 2024 May 30;11:1395721. doi: 10.3389/fmolb.2024.1395721. eCollection 2024. Front Mol Biosci. 2024. PMID: 38872916 Free PMC article.
-
The Biological Roles and Molecular Mechanisms of Long Non-Coding RNA MEG3 in the Hallmarks of Cancer.Cancers (Basel). 2022 Dec 7;14(24):6032. doi: 10.3390/cancers14246032. Cancers (Basel). 2022. PMID: 36551518 Free PMC article. Review.
-
MEG3-Mediated Oral Squamous-Cell-Carcinoma-Derived Exosomal miR-421 Activates Angiogenesis by Targeting HS2ST1 in Vascular Endothelial Cells.Int J Mol Sci. 2024 Jul 10;25(14):7576. doi: 10.3390/ijms25147576. Int J Mol Sci. 2024. PMID: 39062818 Free PMC article.
References
-
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69:7‐34. - PubMed
-
- Rahimy E, Kuo SZ, Ongkeko WM. Evaluation of non‐coding RNAs as potential targets in head and neck squamous cell carcinoma cancer stem cells. Curr Drug Targets. 2014;15:1247‐1260. - PubMed
-
- Dunne S, Mooney O, Coffey L, et al. Psychological variables associated with quality of life following primary treatment for head and neck cancer: a systematic review of the literature from 2004 to 2015. Psychooncology. 2017;26:149‐160. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

