Human PCNA Structure, Function and Interactions
- PMID: 32276417
- PMCID: PMC7225939
- DOI: 10.3390/biom10040570
Human PCNA Structure, Function and Interactions
Abstract
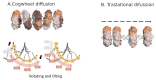
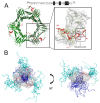
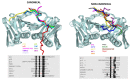
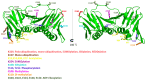
: Proliferating cell nuclear antigen (PCNA) is an essential factor in DNA replication and repair. It forms a homotrimeric ring that embraces the DNA and slides along it, anchoring DNA polymerases and other DNA editing enzymes. It also interacts with regulatory proteins through a sequence motif known as PCNA Interacting Protein box (PIP-box). We here review the latest contributions to knowledge regarding the structure-function relationships in human PCNA, particularly the mechanism of sliding, and of the molecular recognition of canonical and non-canonical PIP motifs. The unique binding mode of the oncogene p15 is described in detail, and the implications of the recently discovered structure of PCNA bound to polymerase δ are discussed. The study of the post-translational modifications of PCNA and its partners may yield therapeutic opportunities in cancer treatment, in addition to illuminating the way PCNA coordinates the dynamic exchange of its many partners in DNA replication and repair.
Keywords: DNA repair; DNA replication; DNA sliding; PCNA; molecular recognition; protein interactions; structure.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures


Similar articles
-
Structural basis for novel interactions between human translesion synthesis polymerases and proliferating cell nuclear antigen.J Biol Chem. 2009 Apr 17;284(16):10552-60. doi: 10.1074/jbc.M809745200. Epub 2009 Feb 10. J Biol Chem. 2009. PMID: 19208623 Free PMC article.
-
PCNA molecular recognition of different PIP motifs: Role of Tyr211 phosphorylation.Int J Biol Macromol. 2024 Jul;273(Pt 2):133187. doi: 10.1016/j.ijbiomac.2024.133187. Epub 2024 Jun 14. Int J Biol Macromol. 2024. PMID: 38880460
-
Structure of p15(PAF)-PCNA complex and implications for clamp sliding during DNA replication and repair.Nat Commun. 2015 Mar 12;6:6439. doi: 10.1038/ncomms7439. Nat Commun. 2015. PMID: 25762514
-
Targeting PCNA with Peptide Mimetics for Therapeutic Purposes.Chembiochem. 2020 Feb 17;21(4):442-450. doi: 10.1002/cbic.201900275. Epub 2019 Oct 15. Chembiochem. 2020. PMID: 31247123 Review.
-
Proliferating cell nuclear antigen structure and interactions: too many partners for one dancer?Adv Protein Chem Struct Biol. 2013;91:1-36. doi: 10.1016/B978-0-12-411637-5.00001-9. Adv Protein Chem Struct Biol. 2013. PMID: 23790209 Review.
Cited by
-
Astragaloside IV Treats Parkinson's Disease by Regulating the Proliferation and Differentiation of NSCs through the SHH-Nurr1 Pathway.Stem Cells Int. 2024 Sep 3;2024:2792909. doi: 10.1155/2024/2792909. eCollection 2024. Stem Cells Int. 2024. PMID: 39257865 Free PMC article.
-
Cytoplasmic PCNA is located in the actin belt and involved in osteoclast differentiation.Aging (Albany NY). 2020 Jun 27;12(13):13297-13317. doi: 10.18632/aging.103434. Epub 2020 Jun 27. Aging (Albany NY). 2020. PMID: 32597793 Free PMC article.
-
20(S)-ginsenoside Rg3 exerts anti-fibrotic effect after myocardial infarction by alleviation of fibroblasts proliferation and collagen deposition through TGFBR1 signaling pathways.J Ginseng Res. 2023 Nov;47(6):743-754. doi: 10.1016/j.jgr.2023.06.007. Epub 2023 Jul 3. J Ginseng Res. 2023. PMID: 38107395 Free PMC article.
-
Melatonin Modulates Cell Cycle Dynamics and Promotes Hippocampal Cell Proliferation After Ischemic Injury in Neonatal Rats.Mol Neurobiol. 2024 Sep;61(9):6910-6919. doi: 10.1007/s12035-024-04013-x. Epub 2024 Feb 15. Mol Neurobiol. 2024. PMID: 38358438 Free PMC article.
-
Hypoxia Inhibits Cell Cycle Progression and Cell Proliferation in Brain Microvascular Endothelial Cells via the miR-212-3p/MCM2 Axis.Int J Mol Sci. 2023 Feb 1;24(3):2788. doi: 10.3390/ijms24032788. Int J Mol Sci. 2023. PMID: 36769104 Free PMC article.
References
-
- Stukenberg P.T., Studwell-Vaughan P.S., O’Donnell M. Mechanism of the sliding β-clamp of DNA polymerase III holoenzyme. J. Biol. Chem. 1991;266:11328–11334. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

