The Mtb-HIV syndemic interaction: why treating M. tuberculosis infection may be crucial for HIV-1 eradication
- PMID: 32273900
- PMCID: PMC7132588
- DOI: 10.2217/fvl-2019-0069
The Mtb-HIV syndemic interaction: why treating M. tuberculosis infection may be crucial for HIV-1 eradication
Abstract
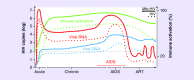
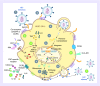
Accelerated tuberculosis and AIDS progression seen in HIV-1 and Mycobacterium tuberculosis (Mtb)-coinfected individuals indicates the important interaction between these syndemic pathogens. The immunological interaction between HIV-1 and Mtb has been largely defined by how the virus exacerbates tuberculosis disease pathogenesis. Understanding of the mechanisms by which pre-existing or subsequent Mtb infection may favor the replication, persistence and progression of HIV, is less characterized. We present a rationale for the critical consideration of 'latent' Mtb infection in HIV-1 prevention and cure strategies. In support of this position, we review evidence of the effect of Mtb infection on HIV-1 acquisition, replication and persistence. We propose that 'latent' Mtb infection may have considerable impact on HIV-1 pathogenesis and the continuing HIV-1 epidemic in sub-Saharan Africa.
Keywords: AIDS; HIV-1 cure; granuloma; immune activation; latency; transmission; tuberculosis; viral expansion; viral reservoir.
© 2020 Anna Coussens.
Conflict of interest statement
Financial & competing interests disclosure R Waters is supported by the Dept Orthopaedic Surgery (UCT) PhD Scholarship; M Ndengane is supported by the South African National Research Foundation (NRF) PhD Scholarship; CR Diedrich is supported by NIH AI 134195; MR Abrahams is supported by the South African Department of Higher Education and Training’s New Generation of Academics Programme; RJ Wilkinson is supported by the Francis Crick Institute, which receives funding from the Cancer Research (UK) (10218), and Wellcome (10218) UKRI (10218) and by Wellcome (104803, 203135); AK Coussens is supported by the Walter and Eliza Hall Institute of Medical Research, the Medical Research Council of South Africa (SHIP-02-2013), the National Institute of Health TB Research Unit (U19AI111276) and the NRF (UID109040). The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed. No writing assistance was utilized in the production of this manuscript.
Figures

Similar articles
-
Tuberculosis.In: Holmes KK, Bertozzi S, Bloom BR, Jha P, editors. Major Infectious Diseases. 3rd edition. Washington (DC): The International Bank for Reconstruction and Development / The World Bank; 2017 Nov 3. Chapter 11. In: Holmes KK, Bertozzi S, Bloom BR, Jha P, editors. Major Infectious Diseases. 3rd edition. Washington (DC): The International Bank for Reconstruction and Development / The World Bank; 2017 Nov 3. Chapter 11. PMID: 30212088 Free Books & Documents. Review.
-
HIV/Mtb Co-Infection: From the Amplification of Disease Pathogenesis to an "Emerging Syndemic".Microorganisms. 2023 Mar 27;11(4):853. doi: 10.3390/microorganisms11040853. Microorganisms. 2023. PMID: 37110276 Free PMC article. Review.
-
Effect of Mycobacterium tuberculosis on HIV replication. Role of immune activation.J Immunol. 1996 Aug 1;157(3):1271-8. J Immunol. 1996. PMID: 8757635
-
Pathogenesis of Human Immunodeficiency Virus-Mycobacterium tuberculosis Co-Infection.J Clin Med. 2020 Nov 6;9(11):3575. doi: 10.3390/jcm9113575. J Clin Med. 2020. PMID: 33172001 Free PMC article. Review.
-
HIV Interferes with Mycobacterium tuberculosis Antigen Presentation in Human Dendritic Cells.Am J Pathol. 2016 Dec;186(12):3083-3093. doi: 10.1016/j.ajpath.2016.08.003. Epub 2016 Oct 13. Am J Pathol. 2016. PMID: 27746182
Cited by
-
Cystatin F Depletion in Mycobacterium tuberculosis-Infected Macrophages Improves Cathepsin C/Granzyme B-Driven Cytotoxic Effects on HIV-Infected Cells during Coinfection.Int J Mol Sci. 2024 Jul 26;25(15):8141. doi: 10.3390/ijms25158141. Int J Mol Sci. 2024. PMID: 39125711 Free PMC article.
-
Mycobacterium tuberculosis disease associates with higher HIV-1-specific antibody responses.iScience. 2023 Apr 10;26(5):106631. doi: 10.1016/j.isci.2023.106631. eCollection 2023 May 19. iScience. 2023. PMID: 37168567 Free PMC article.
-
T Cell Responses during Human Immunodeficiency Virus/Mycobacterium tuberculosis Coinfection.Vaccines (Basel). 2024 Aug 9;12(8):901. doi: 10.3390/vaccines12080901. Vaccines (Basel). 2024. PMID: 39204027 Free PMC article. Review.
-
People Living With HIV Have More Intact HIV DNA in Circulating CD4+ T Cells if They Have History of Pulmonary Tuberculosis.Pathog Immun. 2024 Sep 23;9(2):172-193. doi: 10.20411/pai.v9i2.722. eCollection 2024. Pathog Immun. 2024. PMID: 39345793 Free PMC article.
-
Decreased IL-1 β Secretion as a Potential Predictor of Tuberculosis Recurrence in Individuals Diagnosed with HIV.Biomedicines. 2024 Apr 25;12(5):954. doi: 10.3390/biomedicines12050954. Biomedicines. 2024. PMID: 38790916 Free PMC article.
References
-
- Joint United Nations Programme on HIV/AIDS. Global AIDS Update: Miles to Go. UNAIDS, Geneva, Switzerland: (2018).
-
- UNAIDS. Global HIV & AIDS statistics – 2019 fact sheet. (2019). www.unaids.org/en/resources/fact-sheet
-
- Joint United Nations Programme on HIV/AIDS. UNAIDS Warns that Progress is Slowing and Time is Running Out to Reach the 2020 HIV Targets. UNAIDS, Geneva, Switzerland: (2018).
-
- WHO. Global tuberculosis report. (2019). www.who.int/tb/publications/global_report/en/
-
- Glynn JR. Resurgence of tuberculosis and the impact of HIV infection. Br. Med. Bull. 54(3), 579–593 (1998). - PubMed
Publication types
LinkOut - more resources
Full Text Sources
