Randomized Double-Blind Phase II Study of Maintenance Pembrolizumab Versus Placebo After First-Line Chemotherapy in Patients With Metastatic Urothelial Cancer
- PMID: 32271672
- PMCID: PMC7255983
- DOI: 10.1200/JCO.19.03091
Randomized Double-Blind Phase II Study of Maintenance Pembrolizumab Versus Placebo After First-Line Chemotherapy in Patients With Metastatic Urothelial Cancer
Abstract
Purpose: Platinum-based chemotherapy for first-line treatment of metastatic urothelial cancer is typically administered for a fixed duration followed by observation until progression. "Switch maintenance" therapy with PD-1 blockade at the time of chemotherapy cessation may be attractive for mechanistic and pragmatic reasons.
Patients and methods: Patients with metastatic urothelial cancer achieving at least stable disease on first-line platinum-based chemotherapy were enrolled. Patients were randomly assigned double-blind 1:1 to switch maintenance pembrolizumab 200 mg intravenously once every 3 weeks versus placebo for up to 24 months. Patients with disease progression on placebo could cross over to pembrolizumab. The primary objective was to determine the progression-free survival. Secondary objectives included determining overall survival as well as treatment outcomes according to PD-L1 combined positive score (CPS).
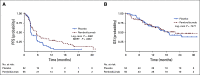
Results: Between December 2015 and November 2018, 108 patients were randomly assigned to pembrolizumab (n = 55) or placebo (n = 53). The objective response rate was 23% with pembrolizumab and 10% with placebo. Treatment-emergent grade 3-4 adverse events occurred in 59% receiving pembrolizumab and 38% of patients receiving placebo. Progression-free survival was significantly longer with maintenance pembrolizumab versus placebo (5.4 months [95% CI, 3.1 to 7.3 months] v 3.0 months [95% CI; 2.7 to 5.5 months]; hazard ratio, 0.65; log-rank P = .04; maximum efficiency robust test P = .039). Median overall survival was 22 months (95% CI, 12.9 months to not reached) with pembrolizumab and 18.7 months (95% CI, 11.4 months to not reached) with placebo. There was no significant interaction between PD-L1 CPS ≥ 10 and treatment arm for progression-free survival or overall survival.
Conclusion: Switch maintenance pembrolizumab leads to additional objective responses in patients achieving at least stable disease with first-line platinum-based chemotherapy and prolongs progression-free survival in patients with metastatic urothelial cancer.
Trial registration: ClinicalTrials.gov NCT02500121.
Figures
Similar articles
-
Durvalumab alone and durvalumab plus tremelimumab versus chemotherapy in previously untreated patients with unresectable, locally advanced or metastatic urothelial carcinoma (DANUBE): a randomised, open-label, multicentre, phase 3 trial.Lancet Oncol. 2020 Dec;21(12):1574-1588. doi: 10.1016/S1470-2045(20)30541-6. Epub 2020 Sep 21. Lancet Oncol. 2020. PMID: 32971005 Clinical Trial.
-
Pembrolizumab alone or combined with chemotherapy versus chemotherapy as first-line therapy for advanced urothelial carcinoma (KEYNOTE-361): a randomised, open-label, phase 3 trial.Lancet Oncol. 2021 Jul;22(7):931-945. doi: 10.1016/S1470-2045(21)00152-2. Epub 2021 May 26. Lancet Oncol. 2021. PMID: 34051178 Clinical Trial.
-
Pembrolizumab plus chemotherapy versus chemotherapy alone for first-line treatment of advanced oesophageal cancer (KEYNOTE-590): a randomised, placebo-controlled, phase 3 study.Lancet. 2021 Aug 28;398(10302):759-771. doi: 10.1016/S0140-6736(21)01234-4. Lancet. 2021. PMID: 34454674 Clinical Trial.
-
Pembrolizumab for Previously Treated Advanced or Metastatic Urothelial Cancer: An Evidence Review Group Perspective of a NICE Single Technology Appraisal.Pharmacoeconomics. 2019 Jan;37(1):19-27. doi: 10.1007/s40273-018-0689-3. Pharmacoeconomics. 2019. PMID: 30030817 Review.
-
Pembrolizumab in the preoperative setting of triple-negative breast cancer: safety and efficacy.Expert Rev Anticancer Ther. 2020 Nov;20(11):923-930. doi: 10.1080/14737140.2020.1823224. Epub 2020 Oct 31. Expert Rev Anticancer Ther. 2020. PMID: 32930616 Review.
Cited by
-
Cell death-induced immunogenicity enhances chemoimmunotherapeutic response by converting immune-excluded into T-cell inflamed bladder tumors.Nat Commun. 2022 Mar 28;13(1):1487. doi: 10.1038/s41467-022-29026-9. Nat Commun. 2022. PMID: 35347124 Free PMC article.
-
Pan-cancer analysis of heterogeneity of tumor mutational burden and genomic mutation under treatment pressure.ESMO Open. 2024 Jul;9(7):103494. doi: 10.1016/j.esmoop.2024.103494. Epub 2024 Jul 8. ESMO Open. 2024. PMID: 38981309 Free PMC article.
-
Cardiovascular toxicity following immune checkpoint inhibitors: A systematic review and meta-analysis.Transl Oncol. 2022 May;19:101383. doi: 10.1016/j.tranon.2022.101383. Epub 2022 Mar 3. Transl Oncol. 2022. PMID: 35248919 Free PMC article.
-
Impact of performance status on treatment outcomes: A real-world study of advanced urothelial cancer treated with immune checkpoint inhibitors.Cancer. 2020 Mar 15;126(6):1208-1216. doi: 10.1002/cncr.32645. Epub 2019 Dec 12. Cancer. 2020. PMID: 31829450 Free PMC article.
-
Precision Medicine to Treat Urothelial Carcinoma-The Way Forward.Cancers (Basel). 2023 Jun 1;15(11):3024. doi: 10.3390/cancers15113024. Cancers (Basel). 2023. PMID: 37296985 Free PMC article. Review.
References
-
- von der Maase H, Sengelov L, Roberts JT, et al: Long-term survival results of a randomized trial comparing gemcitabine plus cisplatin, with methotrexate, vinblastine, doxorubicin, plus cisplatin in patients with bladder cancer. J Clin Oncol 23:4602-4608, 2005. - PubMed
-
- Galsky MD, Hahn NM, Rosenberg J, et al: Treatment of patients with metastatic urothelial cancer “unfit” for cisplatin-based chemotherapy. J Clin Oncol 29:2432-2438, 2011. - PubMed
-
- von der Maase H, Hansen SW, Roberts JT, et al. Gemcitabine and cisplatin versus methotrexate, vinblastine, doxorubicin, and cisplatin in advanced or metastatic bladder cancer: Results of a large, randomized, multinational, multicenter, phase III study. J Clin Oncol. 2000;18:3068–3077. - PubMed
-
- Grivas PD, Daignault S, Tagawa ST, et al. Double-blind, randomized, phase 2 trial of maintenance sunitinib versus placebo after response to chemotherapy in patients with advanced urothelial carcinoma. Cancer. 2014;120:692–701. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

