Cyanobacteria-Based Bio-Oxygen Pump Promoting Hypoxia-Resistant Photodynamic Therapy
- PMID: 32266251
- PMCID: PMC7105637
- DOI: 10.3389/fbioe.2020.00237
Cyanobacteria-Based Bio-Oxygen Pump Promoting Hypoxia-Resistant Photodynamic Therapy
Abstract
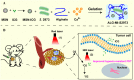
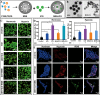
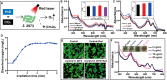
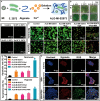
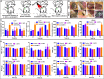
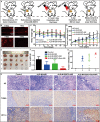
Hypoxia not only alters tumor microenvironment but leads to the tumor progression and metastasis as well as drug resistance. As a promising strategy, photodynamic therapy (PDT) can inhibit tumor by catalyzing O2 to cytotoxic reactive oxygen species. However, its effects were limited by hypoxia and in turn deteriorate hypoxia due to O2 consumption. Hereon, aiming to alleviate hypoxia and promote PDT, a bio-oxygen pump was created based on cyanobacteria, which are the only prokaryotic organisms performing oxygenic photosynthesis. Detailly, controlled-release PDT via loading indocyanine green into mesoporous silica nanoparticles was established. Then bio-oxygen pump based on a fast-growing cyanobacterium Synechococcus elongatus UTEX 2973 was tested and further packaged together with PDT to create an injectable hydrogel. The packaged hydrogel showed stable oxygen production and synergetic therapy effect especially toward hypoxia 4T1 cells in vitro. More importantly, strong in vivo therapeutic effect reaching almost 100% inhibition on tumor tissues was realized using PDT equipped with oxygen pump, with only negligible in vivo side effect on healthy mice from S. elongatus UTEX 2973. The new photo-oxygen-dynamic therapy presented here provided a promising strategy against hypoxia-resistant tumor and may worth further modifications for therapeutic application.
Keywords: hypoxia; indocyanine green; injectable hydrogels; nanoparticles; oxygenic cyanobacteria; photodynamic therapy.
Copyright © 2020 Sun, Zhang, Zhang, Wang, Pan, Liu, Li, Chen, Chang and Zhang.
Figures
Similar articles
-
An injectable hydrogel co-loading with cyanobacteria and upconversion nanoparticles for enhanced photodynamic tumor therapy.Colloids Surf B Biointerfaces. 2021 May;201:111640. doi: 10.1016/j.colsurfb.2021.111640. Epub 2021 Feb 18. Colloids Surf B Biointerfaces. 2021. PMID: 33640676
-
Synchronous delivery of oxygen and photosensitizer for alleviation of hypoxia tumor microenvironment and dramatically enhanced photodynamic therapy.Drug Deliv. 2018 Nov;25(1):585-599. doi: 10.1080/10717544.2018.1435751. Drug Deliv. 2018. PMID: 29461122 Free PMC article.
-
Prodrug and Glucose Oxidase Coloaded Photodynamic Hydrogels for Combinational Therapy of Melanoma.ACS Biomater Sci Eng. 2022 Nov 14;8(11):4886-4895. doi: 10.1021/acsbiomaterials.2c00992. Epub 2022 Oct 24. ACS Biomater Sci Eng. 2022. PMID: 36278808
-
Recent Strategies to Address Hypoxic Tumor Environments in Photodynamic Therapy.Pharmaceutics. 2022 Aug 24;14(9):1763. doi: 10.3390/pharmaceutics14091763. Pharmaceutics. 2022. PMID: 36145513 Free PMC article. Review.
-
Manipulating tumor hypoxia toward enhanced photodynamic therapy (PDT).Biomater Sci. 2017 Jul 25;5(8):1500-1511. doi: 10.1039/c7bm00392g. Biomater Sci. 2017. PMID: 28681887 Review.
Cited by
-
Towards chlorocytes for therapeutic intravascular photosynthesis.Appl Microbiol Biotechnol. 2024 Oct 17;108(1):489. doi: 10.1007/s00253-024-13285-1. Appl Microbiol Biotechnol. 2024. PMID: 39417888 Free PMC article. Review.
-
The development of live microorganism-based oxygen shuttles for enhanced hypoxic tumor therapy.Mater Today Bio. 2022 Dec 10;18:100517. doi: 10.1016/j.mtbio.2022.100517. eCollection 2023 Feb. Mater Today Bio. 2022. PMID: 36578285 Free PMC article. Review.
-
Application of Nanoparticles in the Diagnosis and Treatment of Colorectal Cancer.Anticancer Agents Med Chem. 2024;24(18):1305-1326. doi: 10.2174/0118715206323900240807110122. Anticancer Agents Med Chem. 2024. PMID: 39129164 Free PMC article. Review.
-
Mesoporous Materials Make Hydrogels More Powerful in Biomedicine.Gels. 2023 Mar 9;9(3):207. doi: 10.3390/gels9030207. Gels. 2023. PMID: 36975656 Free PMC article. Review.
-
Microalgae share key features with human erythrocytes and can safely circulate through the vascular system in mice.Appl Microbiol Biotechnol. 2023 Jul;107(14):4621-4633. doi: 10.1007/s00253-023-12588-z. Epub 2023 May 25. Appl Microbiol Biotechnol. 2023. PMID: 37227473
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

