Long-Term Protection Elicited by a DNA Vaccine Candidate Expressing the prM-E Antigen of Dengue Virus Serotype 3 in Mice
- PMID: 32257963
- PMCID: PMC7089926
- DOI: 10.3389/fcimb.2020.00087
Long-Term Protection Elicited by a DNA Vaccine Candidate Expressing the prM-E Antigen of Dengue Virus Serotype 3 in Mice
Abstract
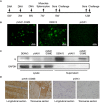
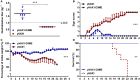
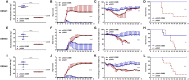
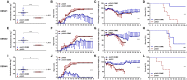
Dengue virus (DENV) is the causative agent of dengue, and its incidence has increased 30-fold in the past five decades. Among the four cocirculating serotypes, DENV3 is associated with an increased number of severe infections and has become widespread. Vaccination is the mainstay of prevention in reducing disease burden. Previously, the protective efficacy of DNA vaccine candidates toward DENV1, 2, and 4 was confirmed in mice. In this study, a DNA vaccine candidate (pVAX1-D3ME) expressing the prM and E proteins of DENV3 was constructed, and then the immunogenicity and protection were assessed in mice to further develop a tetravalent dengue vaccine. Moreover, the cross-reactive immune responses against the other three serotypes were investigated. The results showed that three doses of 50 μg of pVAX1-D3ME were sufficient to induce strong antigen-specific T cell responses and robust and consistent neutralizing antibodies. Additionally, immunization with pVAX1-D3ME offered protective immunity against not only DENV3 but also the other three serotypes, which could be observed even after 12 months. This study shows great promise for the further evaluation of a dengue tetravalent DNA vaccine candidate in large animal models, including non-human primates.
Keywords: DNA vaccine; cross-protection; dengue virus; immunization; prM-E.
Copyright © 2020 Feng, Zheng, Wang, Gao, Fan, Sheng, Zhou, Chen and An.
Figures



Similar articles
-
Electroporation-Mediated Immunization of a Candidate DNA Vaccine Expressing Dengue Virus Serotype 4 prM-E Antigen Confers Long-Term Protection in Mice.Virol Sin. 2019 Feb;34(1):88-96. doi: 10.1007/s12250-019-00090-8. Epub 2019 Feb 21. Virol Sin. 2019. PMID: 30790202 Free PMC article.
-
A Dengue Virus Serotype 1 mRNA-LNP Vaccine Elicits Protective Immune Responses.J Virol. 2021 May 24;95(12):e02482-20. doi: 10.1128/JVI.02482-20. Print 2021 May 24. J Virol. 2021. PMID: 33762420 Free PMC article.
-
Virus-like particle secretion and genotype-dependent immunogenicity of dengue virus serotype 2 DNA vaccine.J Virol. 2014 Sep;88(18):10813-30. doi: 10.1128/JVI.00810-14. Epub 2014 Jul 9. J Virol. 2014. PMID: 25008922 Free PMC article.
-
Dengue Vaccines: The Promise and Pitfalls of Antibody-Mediated Protection.Cell Host Microbe. 2021 Jan 13;29(1):13-22. doi: 10.1016/j.chom.2020.12.011. Cell Host Microbe. 2021. PMID: 33444553 Review.
-
Identification and selection of immunodominant B and T cell epitopes for dengue multi-epitope-based vaccine.Med Microbiol Immunol. 2021 Feb;210(1):1-11. doi: 10.1007/s00430-021-00700-x. Epub 2021 Jan 30. Med Microbiol Immunol. 2021. PMID: 33515283 Review.
Cited by
-
An Intramuscular DNA Vaccine for SARS-CoV-2 Decreases Viral Lung Load but Not Lung Pathology in Syrian Hamsters.Microorganisms. 2021 May 12;9(5):1040. doi: 10.3390/microorganisms9051040. Microorganisms. 2021. PMID: 34065996 Free PMC article.
-
Nucleic Acid Vaccine Platform for DENGUE and ZIKA Flaviviruses.Vaccines (Basel). 2022 May 24;10(6):834. doi: 10.3390/vaccines10060834. Vaccines (Basel). 2022. PMID: 35746442 Free PMC article. Review.
-
Mice with type I interferon signaling deficiency are prone to epilepsy upon HSV-1 infection.Virol Sin. 2024 Apr;39(2):251-263. doi: 10.1016/j.virs.2024.01.002. Epub 2024 Jan 14. Virol Sin. 2024. PMID: 38219860 Free PMC article.
-
Dengue Virus and Vaccines: How Can DNA Immunization Contribute to This Challenge?Front Med Technol. 2021 Apr 12;3:640964. doi: 10.3389/fmedt.2021.640964. eCollection 2021. Front Med Technol. 2021. PMID: 35047911 Free PMC article. Review.
-
Yersinia pestis Plasminogen Activator.Biomolecules. 2020 Nov 14;10(11):1554. doi: 10.3390/biom10111554. Biomolecules. 2020. PMID: 33202679 Free PMC article. Review.
References
-
- Bustos-Arriaga J., Gromowski G. D., Tsetsarkin K. A., Firestone C. Y., Castro-Jimenez T., Pletnev A. G., et al. . (2018). Decreased accumulation of subgenomic RNA in human cells infected with vaccine candidate DEN4Delta30 increases viral susceptibility to type I interferon. Vaccine 36, 3460–3467. 10.1016/j.vaccine.2018.04.087 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

