Management of Adenoviral Keratoconjunctivitis: Challenges and Solutions
- PMID: 32256043
- PMCID: PMC7094151
- DOI: 10.2147/OPTH.S207976
Management of Adenoviral Keratoconjunctivitis: Challenges and Solutions
Abstract
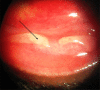
Human adenovirus (HAdV) is the most common cause of infectious conjunctivitis, accounting for up to 75% of all conjunctivitis cases and affecting people of all ages and demographics. In addition to ocular complications, it can cause systemic infections in the form of gastroenteritis, respiratory disease, and dissemination in immunocompromised individuals. HAdV causes lytic infection of the mucoepithelial cells of the conjunctiva and cornea, as well as latent infection of lymphoid and adenoid cells. Epidemic keratoconjunctivitis (EKC) is the most severe ocular manifestation of HAdV infection, in which the presence of subepithelial infiltrates (SEIs) in the cornea is a hallmark feature of corneal involvement. SEIs have the tendency to recur and may lead to long-term visual disability. HAdV persistence and dissemination are linked to sporadic outbreaks of adenoviral keratoconjunctivitis. There is no FDA-approved antiviral for treating adenoviral keratoconjunctivitis, and as such, solutions should be proffered to handle the challenges associated with viral persistence and dissemination. Several treatment modalities have been investigated, both systemically and locally, to not only mitigate symptoms but reduce the course of the infection and prevent the risk of long-term complications. These options include systemic and topical antivirals, in-office povidone-iodine irrigation (PVI), immunoglobulin-based therapy, anti-inflammatory therapy, and immunotherapy. More recently, combination PVI/dexamethasone ophthalmic formulations have shown favorable outcomes and were well tolerated in clinical trials for the treatment of EKC. Possible, future treatment considerations include sialic acid analogs, cold atmospheric plasma, N-chlorotaurine, and benzalkonium chloride. Continued investigation and evaluation of treatment are warranted to reduce the economic burden and potential long-term visual debilitation in affected patients. This review will focus on how persistence and dissemination of HAdV pose a significant challenge to the management of adenoviral keratoconjunctivitis. Furthermore, current and future trends in prophylactic and therapeutic modalities for adenoviral keratoconjunctivitis will be discussed.
Keywords: adenoviral keratoconjunctivitis; antivirals; human adenovirus; immunotherapy; povidone-iodine; viral dissemination.
© 2020 Labib et al.
Conflict of interest statement
The authors report no financial affiliations or conflicts of interest in this work.
Figures

Similar articles
-
Pathogenesis and management of adenoviral keratoconjunctivitis.Infect Drug Resist. 2018 Jul 17;11:981-993. doi: 10.2147/IDR.S162669. eCollection 2018. Infect Drug Resist. 2018. PMID: 30046247 Free PMC article. Review.
-
Epidemic Keratoconjunctivitis-Causing Adenoviruses Induce MUC16 Ectodomain Release To Infect Ocular Surface Epithelial Cells.mSphere. 2016 Feb 10;1(1):e00112-15. doi: 10.1128/mSphere.00112-15. eCollection 2016 Jan-Feb. mSphere. 2016. PMID: 27303700 Free PMC article.
-
Mystery eye: Human adenovirus and the enigma of epidemic keratoconjunctivitis.Prog Retin Eye Res. 2020 May;76:100826. doi: 10.1016/j.preteyeres.2019.100826. Epub 2019 Dec 28. Prog Retin Eye Res. 2020. PMID: 31891773 Free PMC article. Review.
-
Molecular, Epidemiological and Clinical Assessment of Adenoviral Keratoconjunctivitis in Egypt: Institutional Study.Ocul Immunol Inflamm. 2023 Oct;31(8):1640-1646. doi: 10.1080/09273948.2022.2092004. Epub 2022 Jul 11. Ocul Immunol Inflamm. 2023. PMID: 35816022
-
Challenges in management of epidemic keratoconjunctivitis with emerging recombinant human adenoviruses.J Clin Virol. 2019 Mar;112:1-9. doi: 10.1016/j.jcv.2019.01.004. Epub 2019 Jan 11. J Clin Virol. 2019. PMID: 30654207 Review.
Cited by
-
Epidemiologic and clinical updates on viral infections in Saudi Arabia.Saudi Pharm J. 2024 Jul;32(7):102126. doi: 10.1016/j.jsps.2024.102126. Epub 2024 Jun 8. Saudi Pharm J. 2024. PMID: 38966679 Free PMC article.
-
Topical Pharmacologic Interventions Versus Active Control, Placebo, or No Treatment for Epidemic Keratoconjunctivitis: Findings From a Cochrane Systematic Review.Am J Ophthalmol. 2022 Aug;240:265-275. doi: 10.1016/j.ajo.2022.03.018. Epub 2022 Mar 22. Am J Ophthalmol. 2022. PMID: 35331686 Free PMC article. Review.
-
Virucidal benefits of povidone-iodine use on the ocular surface: a review.BMJ Open Ophthalmol. 2020 Aug 4;5(1):e000509. doi: 10.1136/bmjophth-2020-000509. eCollection 2020. BMJ Open Ophthalmol. 2020. PMID: 32818151 Free PMC article. Review.
-
Topical pharmacologic interventions versus placebo for epidemic keratoconjunctivitis.Cochrane Database Syst Rev. 2022 Mar 3;3(3):CD013520. doi: 10.1002/14651858.CD013520.pub2. Cochrane Database Syst Rev. 2022. PMID: 35238405 Free PMC article. Review.
-
Characterization of the conserved regions of E1A protein from human adenovirus for reinforcement of cytotoxic T lymphocytes responses to the all genogroups causes ocular manifestation through an in silico approach.Iran J Microbiol. 2022 Oct;14(5):746-758. doi: 10.18502/ijm.v14i5.10971. Iran J Microbiol. 2022. PMID: 36531810 Free PMC article.
References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

