Axonopathy and Reduction of Membrane Resistance: Key Features in a New Murine Model of Human GM1-Gangliosidosis
- PMID: 32252429
- PMCID: PMC7230899
- DOI: 10.3390/jcm9041004
Axonopathy and Reduction of Membrane Resistance: Key Features in a New Murine Model of Human GM1-Gangliosidosis
Abstract
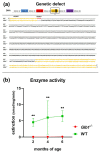
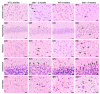
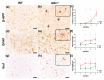
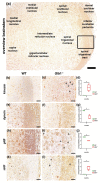
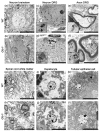
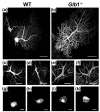
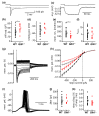
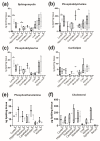
GM1-gangliosidosis is caused by a reduced activity of β-galactosidase (Glb1), resulting in intralysosomal accumulations of GM1. The aim of this study was to reveal the pathogenic mechanisms of GM1-gangliosidosis in a new Glb1 knockout mouse model. Glb1-/- mice were analyzed clinically, histologically, immunohistochemically, electrophysiologically and biochemically. Morphological lesions in the central nervous system were already observed in two-month-old mice, whereas functional deficits, including ataxia and tremor, did not start before 3.5-months of age. This was most likely due to a reduced membrane resistance as a compensatory mechanism. Swollen neurons exhibited intralysosomal storage of lipids extending into axons and amyloid precursor protein positive spheroids. Additionally, axons showed a higher kinesin and lower dynein immunoreactivity compared to wildtype controls. Glb1-/- mice also demonstrated loss of phosphorylated neurofilament positive axons and a mild increase in non-phosphorylated neurofilament positive axons. Moreover, marked astrogliosis and microgliosis were found, but no demyelination. In addition to the main storage material GM1, GA1, sphingomyelin, phosphatidylcholine and phosphatidylserine were elevated in the brain. In summary, the current Glb1-/- mice exhibit a so far undescribed axonopathy and a reduced membrane resistance to compensate the functional effects of structural changes. They can be used for detailed examinations of axon-glial interactions and therapy trials of lysosomal storage diseases.
Keywords: GM1-gangliosidosis; astrogliosis; axonopathy; electrophysiology; knockout mouse model; lipid analysis; microgliosis; neuronal vacuolation; β-galactosidase deficiency.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the study design; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures

Similar articles
-
GM1 Gangliosidosis: Mechanisms and Management.Appl Clin Genet. 2021 Apr 9;14:209-233. doi: 10.2147/TACG.S206076. eCollection 2021. Appl Clin Genet. 2021. PMID: 33859490 Free PMC article. Review.
-
GM1 Gangliosidosis-A Mini-Review.Front Genet. 2021 Sep 3;12:734878. doi: 10.3389/fgene.2021.734878. eCollection 2021. Front Genet. 2021. PMID: 34539759 Free PMC article. Review.
-
Glb1 knockout mouse model shares natural history with type II GM1 gangliosidosis patients.Mol Genet Metab. 2023 Feb;138(2):107508. doi: 10.1016/j.ymgme.2023.107508. Epub 2023 Jan 13. Mol Genet Metab. 2023. PMID: 36709532 Free PMC article.
-
Sialidase NEU3 action on GM1 ganglioside is neuroprotective in GM1 gangliosidosis.J Lipid Res. 2023 Dec;64(12):100463. doi: 10.1016/j.jlr.2023.100463. Epub 2023 Oct 21. J Lipid Res. 2023. PMID: 37871851 Free PMC article.
-
Human GLB1 knockout cerebral organoids: A model system for testing AAV9-mediated GLB1 gene therapy for reducing GM1 ganglioside storage in GM1 gangliosidosis.Mol Genet Metab Rep. 2019 Sep 11;21:100513. doi: 10.1016/j.ymgmr.2019.100513. eCollection 2019 Dec. Mol Genet Metab Rep. 2019. PMID: 31534909 Free PMC article.
Cited by
-
GM1 Gangliosidosis: Mechanisms and Management.Appl Clin Genet. 2021 Apr 9;14:209-233. doi: 10.2147/TACG.S206076. eCollection 2021. Appl Clin Genet. 2021. PMID: 33859490 Free PMC article. Review.
-
Phenotypical changes of satellite glial cells in a murine model of GM1 -gangliosidosis.J Cell Mol Med. 2022 Jan;26(2):527-539. doi: 10.1111/jcmm.17113. Epub 2021 Dec 7. J Cell Mol Med. 2022. PMID: 34877779 Free PMC article.
-
GM1 Gangliosidosis-A Mini-Review.Front Genet. 2021 Sep 3;12:734878. doi: 10.3389/fgene.2021.734878. eCollection 2021. Front Genet. 2021. PMID: 34539759 Free PMC article. Review.
-
Lysosomal positioning diseases: beyond substrate storage.Open Biol. 2022 Oct;12(10):220155. doi: 10.1098/rsob.220155. Epub 2022 Oct 26. Open Biol. 2022. PMID: 36285443 Free PMC article. Review.
-
Polymer-based drug delivery systems under investigation for enzyme replacement and other therapies of lysosomal storage disorders.Adv Drug Deliv Rev. 2023 Jun;197:114683. doi: 10.1016/j.addr.2022.114683. Epub 2023 Jan 16. Adv Drug Deliv Rev. 2023. PMID: 36657645 Free PMC article. Review.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

