The Role of Cytokines including Interleukin-6 in COVID-19 induced Pneumonia and Macrophage Activation Syndrome-Like Disease
- PMID: 32251717
- PMCID: PMC7195002
- DOI: 10.1016/j.autrev.2020.102537
The Role of Cytokines including Interleukin-6 in COVID-19 induced Pneumonia and Macrophage Activation Syndrome-Like Disease
Abstract
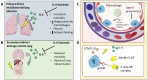
Severe COVID-19 associated pneumonia patients may exhibit features of systemic hyper-inflammation designated under the umbrella term of macrophage activation syndrome (MAS) or cytokine storm, also known as secondary haemophagocytic lymphohistocytosis (sHLH). This is distinct from HLH associated with immunodeficiency states termed primary HLH -with radically different therapy strategies in both situations. COVID-19 infection with MAS typically occurs in subjects with adult respiratory distress syndrome (ARDS) and historically, non-survival in ARDS was linked to sustained IL-6 and IL-1 elevation. We provide a model for the classification of MAS to stratify the MAS-like presentation in COVID-19 pneumonia and explore the complexities of discerning ARDS from MAS. We discuss the potential impact of timing of anti-cytokine therapy on viral clearance and the impact of such therapy on intra-pulmonary macrophage activation and emergent pulmonary vascular disease.
Crown Copyright © 2020. Published by Elsevier B.V. All rights reserved.
Figures


Comment in
-
Tocilizumab for cytokine storm syndrome in COVID-19 pneumonia: an increased risk for candidemia?Autoimmun Rev. 2020 Jul;19(7):102564. doi: 10.1016/j.autrev.2020.102564. Epub 2020 May 5. Autoimmun Rev. 2020. PMID: 32376396 Free PMC article. No abstract available.
-
Cytokine storm syndrome in severe COVID-19.Autoimmun Rev. 2020 Jul;19(7):102562. doi: 10.1016/j.autrev.2020.102562. Epub 2020 May 3. Autoimmun Rev. 2020. PMID: 32376400 Free PMC article. No abstract available.
-
Imatinib might constitute a treatment option for lung involvement in COVID-19.Autoimmun Rev. 2020 Jul;19(7):102565. doi: 10.1016/j.autrev.2020.102565. Epub 2020 May 3. Autoimmun Rev. 2020. PMID: 32376403 Free PMC article. No abstract available.
-
Why judiciously timed anti-IL 6 therapy may be of benefit in severe COVID-19 infection.Autoimmun Rev. 2020 Jul;19(7):102563. doi: 10.1016/j.autrev.2020.102563. Epub 2020 May 5. Autoimmun Rev. 2020. PMID: 32380318 Free PMC article. No abstract available.
Similar articles
-
Storm, typhoon, cyclone or hurricane in patients with COVID-19? Beware of the same storm that has a different origin.RMD Open. 2020 May;6(1):e001295. doi: 10.1136/rmdopen-2020-001295. RMD Open. 2020. PMID: 32423970 Free PMC article.
-
The Society for Immunotherapy of Cancer perspective on regulation of interleukin-6 signaling in COVID-19-related systemic inflammatory response.J Immunother Cancer. 2020 May;8(1):e000930. doi: 10.1136/jitc-2020-000930. J Immunother Cancer. 2020. PMID: 32385146 Free PMC article.
-
Weathering the Cytokine Storm in Susceptible Patients with Severe SARS-CoV-2 Infection.J Allergy Clin Immunol Pract. 2020 Jun;8(6):1798-1801. doi: 10.1016/j.jaip.2020.04.014. Epub 2020 Apr 18. J Allergy Clin Immunol Pract. 2020. PMID: 32311489 Free PMC article. No abstract available.
-
Cytokine storm in COVID-19: pathogenesis and overview of anti-inflammatory agents used in treatment.Clin Rheumatol. 2020 Jul;39(7):2085-2094. doi: 10.1007/s10067-020-05190-5. Epub 2020 May 30. Clin Rheumatol. 2020. PMID: 32474885 Free PMC article. Review.
-
The amount of cytokine-release defines different shades of Sars-Cov2 infection.Exp Biol Med (Maywood). 2020 Jun;245(11):970-976. doi: 10.1177/1535370220928964. Epub 2020 May 28. Exp Biol Med (Maywood). 2020. PMID: 32460624 Free PMC article. Review.
Cited by
-
Guillain-Barré syndrome associated with SARS-CoV-2 infection. A systematic review.Eur J Neurol. 2020 Nov;27(11):2361-2370. doi: 10.1111/ene.14462. Epub 2020 Sep 11. Eur J Neurol. 2020. PMID: 32757404 Free PMC article.
-
A Pathophysiological Perspective on COVID-19's Lethal Complication: From Viremia to Hypersensitivity Pneumonitis-like Immune Dysregulation.Infect Chemother. 2020 Sep;52(3):335-344. doi: 10.3947/ic.2020.52.3.335. Epub 2020 Jul 15. Infect Chemother. 2020. PMID: 32537960 Free PMC article. Review.
-
Effectiveness of corticoid pulses in patients with cytokine storm syndrome induced by SARS-CoV-2 infection.Med Clin (Engl Ed). 2020 Aug 28;155(4):159-161. doi: 10.1016/j.medcle.2020.07.002. Epub 2020 Jul 10. Med Clin (Engl Ed). 2020. PMID: 32835105 Free PMC article.
-
Cerebral microbleeds in patients with COVID-19: is there an inevitable connection?Brain Commun. 2024 Jul 19;6(5):fcae236. doi: 10.1093/braincomms/fcae236. eCollection 2024. Brain Commun. 2024. PMID: 39229491 Free PMC article. Review.
-
Prevalence of COVID-19 and seroprevalence to SARS-CoV-2 in a rheumatologic patient population from a tertiary referral clinic in Israel.Intern Med J. 2021 May;51(5):682-690. doi: 10.1111/imj.15202. Epub 2021 May 12. Intern Med J. 2021. PMID: 33844415 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

