SAMHD1 Functions and Human Diseases
- PMID: 32244340
- PMCID: PMC7232136
- DOI: 10.3390/v12040382
SAMHD1 Functions and Human Diseases
Abstract
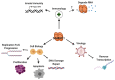
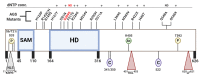
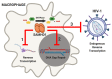
Deoxynucleoside triphosphate (dNTP) molecules are essential for the replication and maintenance of genomic information in both cells and a variety of viral pathogens. While the process of dNTP biosynthesis by cellular enzymes, such as ribonucleotide reductase (RNR) and thymidine kinase (TK), has been extensively investigated, a negative regulatory mechanism of dNTP pools was recently found to involve sterile alpha motif (SAM) domain and histidine-aspartate (HD) domain-containing protein 1, SAMHD1. When active, dNTP triphosphohydrolase activity of SAMHD1 degrades dNTPs into their 2'-deoxynucleoside (dN) and triphosphate subparts, steadily depleting intercellular dNTP pools. The differential expression levels and activation states of SAMHD1 in various cell types contributes to unique dNTP pools that either aid (i.e., dividing T cells) or restrict (i.e., nondividing macrophages) viral replication that consumes cellular dNTPs. Genetic mutations in SAMHD1 induce a rare inflammatory encephalopathy called Aicardi-Goutières syndrome (AGS), which phenotypically resembles viral infection. Recent publications have identified diverse roles for SAMHD1 in double-stranded break repair, genome stability, and the replication stress response through interferon signaling. Finally, a series of SAMHD1 mutations were also reported in various cancer cell types while why SAMHD1 is mutated in these cancer cells remains to investigated. Here, we reviewed a series of studies that have begun illuminating the highly diverse roles of SAMHD1 in virology, immunology, and cancer biology.
Keywords: Aicardi–Goutières syndrome; SAMHD1; cancers; dNTPs; viruses.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
SAMHD1-deficient fibroblasts from Aicardi-Goutières Syndrome patients can escape senescence and accumulate mutations.FASEB J. 2020 Jan;34(1):631-647. doi: 10.1096/fj.201902508R. Epub 2019 Nov 26. FASEB J. 2020. PMID: 31914608
-
SAMHD1: Recurring roles in cell cycle, viral restriction, cancer, and innate immunity.Autoimmunity. 2018 May;51(3):96-110. doi: 10.1080/08916934.2018.1454912. Epub 2018 Mar 27. Autoimmunity. 2018. PMID: 29583030 Free PMC article. Review.
-
Roles of SAMHD1 in antiviral defense, autoimmunity and cancer.Rev Med Virol. 2017 Jul;27(4). doi: 10.1002/rmv.1931. Epub 2017 Apr 25. Rev Med Virol. 2017. PMID: 28444859 Review.
-
SAMHD1-mediated dNTP degradation is required for efficient DNA repair during antibody class switch recombination.EMBO J. 2020 Aug 3;39(15):e102931. doi: 10.15252/embj.2019102931. Epub 2020 Jun 8. EMBO J. 2020. PMID: 32511795 Free PMC article.
-
Unveiling the Connection: Viral Infections and Genes in dNTP Metabolism.Viruses. 2024 Sep 3;16(9):1412. doi: 10.3390/v16091412. Viruses. 2024. PMID: 39339888 Free PMC article. Review.
Cited by
-
Deciphering the role of SAMHD1 in endometrial cancer progression.Biol Direct. 2024 Oct 11;19(1):89. doi: 10.1186/s13062-024-00525-7. Biol Direct. 2024. PMID: 39394602 Free PMC article.
-
SAMHD1 expression is a surrogate marker of immune infiltration and determines prognosis after neoadjuvant chemotherapy in early breast cancer.Cell Oncol (Dordr). 2024 Feb;47(1):189-208. doi: 10.1007/s13402-023-00862-1. Epub 2023 Sep 4. Cell Oncol (Dordr). 2024. PMID: 37667113 Free PMC article.
-
De-ubiquitination of SAMHD1 by USP7 promotes DNA damage repair to overcome oncogenic stress and affect chemotherapy sensitivity.Oncogene. 2023 Jun;42(22):1843-1856. doi: 10.1038/s41388-023-02667-w. Epub 2023 Apr 20. Oncogene. 2023. PMID: 37081042 Free PMC article.
-
Identification and evaluation of small-molecule inhibitors against the dNTPase SAMHD1 via a comprehensive screening funnel.iScience. 2024 Jan 13;27(2):108907. doi: 10.1016/j.isci.2024.108907. eCollection 2024 Feb 16. iScience. 2024. PMID: 38318365 Free PMC article.
-
Metabolic Enzymes in Viral Infection and Host Innate Immunity.Viruses. 2023 Dec 24;16(1):35. doi: 10.3390/v16010035. Viruses. 2023. PMID: 38257735 Free PMC article. Review.
References
-
- Reichard P. Ribonucleotide reductase and deoxyribonucleotide pools. Basic Life Sci. 1985;31:33–45. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

