Rho GTPase Regulators and Effectors in Autism Spectrum Disorders: Animal Models and Insights for Therapeutics
- PMID: 32244264
- PMCID: PMC7226772
- DOI: 10.3390/cells9040835
Rho GTPase Regulators and Effectors in Autism Spectrum Disorders: Animal Models and Insights for Therapeutics
Abstract
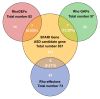
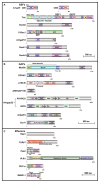
The Rho family GTPases are small G proteins that act as molecular switches shuttling between active and inactive forms. Rho GTPases are regulated by two classes of regulatory proteins, guanine nucleotide exchange factors (GEFs) and GTPase-activating proteins (GAPs). Rho GTPases transduce the upstream signals to downstream effectors, thus regulating diverse cellular processes, such as growth, migration, adhesion, and differentiation. In particular, Rho GTPases play essential roles in regulating neuronal morphology and function. Recent evidence suggests that dysfunction of Rho GTPase signaling contributes substantially to the pathogenesis of autism spectrum disorder (ASD). It has been found that 20 genes encoding Rho GTPase regulators and effectors are listed as ASD risk genes by Simons foundation autism research initiative (SFARI). This review summarizes the clinical evidence, protein structure, and protein expression pattern of these 20 genes. Moreover, ASD-related behavioral phenotypes in animal models of these genes are reviewed, and the therapeutic approaches that show successful treatment effects in these animal models are discussed.
Keywords: GTPase-activating protein; Rho GTPase; animal model; autism spectrum disorder; behavior; guanine nuclear exchange factor.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Structural basis of the Rho GTPase signaling.J Biochem. 2003 Sep;134(3):327-31. doi: 10.1093/jb/mvg149. J Biochem. 2003. PMID: 14561717 Review.
-
Rho GTPase regulatory proteins in podocytes.Kidney Int. 2021 Feb;99(2):336-345. doi: 10.1016/j.kint.2020.08.035. Epub 2020 Oct 26. Kidney Int. 2021. PMID: 33122025 Review.
-
Positive regulation of Rho GTPase activity by RhoGDIs as a result of their direct interaction with GAPs.BMC Syst Biol. 2015 Jan 28;9:3. doi: 10.1186/s12918-015-0143-5. BMC Syst Biol. 2015. PMID: 25628036 Free PMC article.
-
Mechanisms for spatiotemporal regulation of Rho-GTPase signaling at synapses.Neurosci Lett. 2015 Aug 5;601:4-10. doi: 10.1016/j.neulet.2015.05.034. Epub 2015 May 21. Neurosci Lett. 2015. PMID: 26003445 Free PMC article. Review.
-
Biochemical assays to characterize Rho GTPases.Methods Mol Biol. 2012;827:37-58. doi: 10.1007/978-1-61779-442-1_3. Methods Mol Biol. 2012. PMID: 22144266
Cited by
-
P-Rex1 Controls Sphingosine 1-Phosphate Receptor Signalling, Morphology, and Cell-Cycle Progression in Neuronal Cells.Cells. 2021 Sep 18;10(9):2474. doi: 10.3390/cells10092474. Cells. 2021. PMID: 34572121 Free PMC article.
-
Impaired Function of PLEKHG2, a Rho-Guanine Nucleotide-Exchange Factor, Disrupts Corticogenesis in Neurodevelopmental Phenotypes.Cells. 2022 Feb 16;11(4):696. doi: 10.3390/cells11040696. Cells. 2022. PMID: 35203342 Free PMC article.
-
Comparison of chromatin accessibility landscapes during early development of prefrontal cortex between rhesus macaque and human.Nat Commun. 2022 Jul 6;13(1):3883. doi: 10.1038/s41467-022-31403-3. Nat Commun. 2022. PMID: 35794099 Free PMC article.
-
Neurons: The Interplay between Cytoskeleton, Ion Channels/Transporters and Mitochondria.Cells. 2022 Aug 11;11(16):2499. doi: 10.3390/cells11162499. Cells. 2022. PMID: 36010576 Free PMC article. Review.
-
Activation of RhoC by regulatory ubiquitination is mediated by LNX1 and suppressed by LIS1.Sci Rep. 2022 Oct 3;12(1):16493. doi: 10.1038/s41598-022-19740-1. Sci Rep. 2022. PMID: 36192543 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

