Narciclasine, an isocarbostyril alkaloid, has preferential activity against primary effusion lymphoma
- PMID: 32235878
- PMCID: PMC7109099
- DOI: 10.1038/s41598-020-62690-9
Narciclasine, an isocarbostyril alkaloid, has preferential activity against primary effusion lymphoma
Abstract
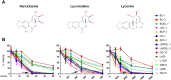
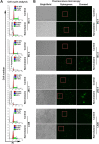
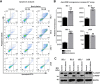
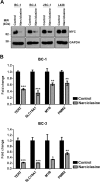
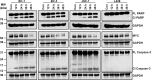
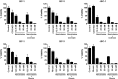
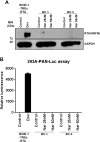
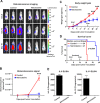
Primary effusion lymphoma (PEL) is a subtype of non-Hodgkin lymphoma associated with infection by Kaposi sarcoma-associated herpes virus (KSHV). PEL is an aggressive disease with extremely poor prognosis when treated with conventional chemotherapy. Narciclasine, a natural product present in Amaryllidaceae family of flowering plants including daffodils, belongs to a class of molecules termed 'isocarbostyril alkaloid'. We have found that narciclasine displays preferential cytotoxicity towards PEL at low nanomolar concentrations and is approximately 10 and 100-fold more potent than its structural analogs lycoricidine and lycorine, respectively. Narciclasine arrested cell-cycle progression at the G1 phase and induced apoptosis in PEL, which is accompanied by activation of caspase-3/7, cleavage of PARP and increase in the surface expression of Annexin-V. Although narciclasine treatment resulted in a marked decrease in the expression of MYC and its direct target genes,time-course experiments revealed that MYC is not a direct target of narciclasine. Narciclasine treatment neither induces the expression of KSHV-RTA/ORF50 nor the production of infectious KSHV virions in PEL. Finally, narciclasine provides dramatic survival advantages to mice in two distinct mouse xenograft models of PEL. In conclusion, our results suggest that narciclasine could be a promising agent for the treatment of PEL.
Conflict of interest statement
The authors declare no competing interests.
Figures
Similar articles
-
Immunomodulatory drugs target IKZF1-IRF4-MYC axis in primary effusion lymphoma in a cereblon-dependent manner and display synergistic cytotoxicity with BRD4 inhibitors.Oncogene. 2016 Apr 7;35(14):1797-810. doi: 10.1038/onc.2015.245. Epub 2015 Jun 29. Oncogene. 2016. PMID: 26119939 Free PMC article.
-
Structure-activity relationship analysis of novel derivatives of narciclasine (an Amaryllidaceae isocarbostyril derivative) as potential anticancer agents.J Med Chem. 2009 Feb 26;52(4):1100-14. doi: 10.1021/jm8013585. J Med Chem. 2009. PMID: 19199649
-
Biscoclaurine alkaloid cepharanthine inhibits the growth of primary effusion lymphoma in vitro and in vivo and induces apoptosis via suppression of the NF-kappaB pathway.Int J Cancer. 2009 Sep 15;125(6):1464-72. doi: 10.1002/ijc.24521. Int J Cancer. 2009. PMID: 19521981
-
Narciclasine as well as other Amaryllidaceae isocarbostyrils are promising GTP-ase targeting agents against brain cancers.Med Res Rev. 2013 Mar;33(2):439-55. doi: 10.1002/med.21253. Epub 2012 Mar 14. Med Res Rev. 2013. PMID: 22419031 Review.
-
Narciclasine - an Amaryllidaceae Alkaloid with Potent Antitumor and Anti-Inflammatory Properties.Planta Med. 2016 Nov;82(16):1389-1394. doi: 10.1055/s-0042-115034. Epub 2016 Aug 19. Planta Med. 2016. PMID: 27542176 Review.
Cited by
-
Chemistry and Biological Activity of Alkaloids from the Genus Lycoris (Amaryllidaceae).Molecules. 2020 Oct 19;25(20):4797. doi: 10.3390/molecules25204797. Molecules. 2020. PMID: 33086636 Free PMC article. Review.
-
Narciclasine is a novel YAP inhibitor that disturbs interaction between YAP and TEAD4.BBA Adv. 2021 Mar 27;1:100008. doi: 10.1016/j.bbadva.2021.100008. eCollection 2021. BBA Adv. 2021. PMID: 37082014 Free PMC article.
-
A novel thermostable beetle luciferase based cytotoxicity assay.Sci Rep. 2021 May 11;11(1):10002. doi: 10.1038/s41598-021-89404-z. Sci Rep. 2021. PMID: 33976304 Free PMC article.
-
Therapeutic targets and pharmacological mechanisms of Coptidis Rhizoma against ulcerative colitis: Findings of system pharmacology and bioinformatics analysis.Front Pharmacol. 2022 Nov 30;13:1037856. doi: 10.3389/fphar.2022.1037856. eCollection 2022. Front Pharmacol. 2022. PMID: 36532769 Free PMC article.
References
-
- Kobayashi Y, et al. Comparison of human herpes virus 8 related primary effusion lymphoma with human herpes virus 8 unrelated primary effusion lymphoma-like lymphoma on the basis of HIV: report of 2 cases and review of 212 cases in the literature. Acta Haematol. 2007;117:132–144. doi: 10.1159/000097460. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

