A Humanized Monoclonal Antibody Cocktail to Prevent Pulmonary Ricin Intoxication
- PMID: 32235318
- PMCID: PMC7232472
- DOI: 10.3390/toxins12040215
A Humanized Monoclonal Antibody Cocktail to Prevent Pulmonary Ricin Intoxication
Abstract
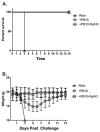
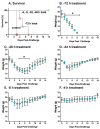
PB10 IgG1, a monoclonal antibody (MAb) directed against an immunodominant epitope on the enzymatic subunit (RTA) of ricin toxin (RT), has been shown to passively protect mice and non-human primates from an aerosolized lethal-dose RT challenge. However, it was recently demonstrated that the therapeutic efficacy of PB10 IgG1 is significantly improved when co-administered with a second MAb, SylH3, targeting RT's binding subunit (RTB). Here we report that the PB10/SylH3 cocktail is also superior to PB10 alone when used as a pre-exposure prophylactic (PrEP) in a mouse model of intranasal RT challenge. The benefit of the PB10/SylH3 cocktail prompted us to engineer a humanized IgG1 version of SylH3 (huSylH3). The huPB10/huSylH3 cocktail proved highly efficacious in the mouse model, thereby opening the door to future testing in non-human primates.
Keywords: antibody; biodefense; lung; prophylactic; toxin.
Conflict of interest statement
M.P., (A.G.), and H.P. are (were) Mapp Biopharmaceutical employees. M.P. is also a shareholder. L.Z. is an employee, shareholder, and co-owner of MappBiopharmaceutical. The other authors declare no conflicts of interest.
Figures
Similar articles
-
An intranasally administered monoclonal antibody cocktail abrogates ricin toxin-induced pulmonary tissue damage and inflammation.Hum Vaccin Immunother. 2020 Apr 2;16(4):793-807. doi: 10.1080/21645515.2019.1664243. Epub 2019 Oct 29. Hum Vaccin Immunother. 2020. PMID: 31589555 Free PMC article.
-
Humanized Monoclonal Antibody That Passively Protects Mice against Systemic and Intranasal Ricin Toxin Challenge.Clin Vaccine Immunol. 2016 Sep 6;23(9):795-9. doi: 10.1128/CVI.00088-16. Print 2016 Sep. Clin Vaccine Immunol. 2016. PMID: 27466351 Free PMC article.
-
Spatial location of neutralizing and non-neutralizing B cell epitopes on domain 1 of ricin toxin's binding subunit.PLoS One. 2017 Jul 10;12(7):e0180999. doi: 10.1371/journal.pone.0180999. eCollection 2017. PLoS One. 2017. PMID: 28700745 Free PMC article.
-
Progress and challenges associated with the development of ricin toxin subunit vaccines.Expert Rev Vaccines. 2016 Sep;15(9):1213-22. doi: 10.1586/14760584.2016.1168701. Epub 2016 Apr 6. Expert Rev Vaccines. 2016. PMID: 26998662 Free PMC article. Review.
-
Treatments for Pulmonary Ricin Intoxication: Current Aspects and Future Prospects.Toxins (Basel). 2017 Oct 3;9(10):311. doi: 10.3390/toxins9100311. Toxins (Basel). 2017. PMID: 28972558 Free PMC article. Review.
Cited by
-
The Search for Antidotes Against Ricin.Mini Rev Med Chem. 2024;24(12):1148-1161. doi: 10.2174/0113895575270509231121060105. Mini Rev Med Chem. 2024. PMID: 38350844 Review.
-
Ricin: an ancient toxicant, but still an evergreen.Arch Toxicol. 2023 Apr;97(4):909-911. doi: 10.1007/s00204-023-03472-w. Epub 2023 Mar 7. Arch Toxicol. 2023. PMID: 36881026 Free PMC article. No abstract available.
-
A Biparatopic Intrabody Renders Vero Cells Impervious to Ricin Intoxication.bioRxiv [Preprint]. 2024 Jul 3:2024.07.02.601761. doi: 10.1101/2024.07.02.601761. bioRxiv. 2024. Update in: Biochemistry. 2024 Oct 1;63(19):2391-2396. doi: 10.1021/acs.biochem.4c00385. PMID: 39005371 Free PMC article. Updated. Preprint.
-
Establishment of a Novel Oral Murine Model of Ricin Intoxication and Efficacy Assessment of Ovine Ricin Antitoxins.Toxins (Basel). 2020 Dec 8;12(12):784. doi: 10.3390/toxins12120784. Toxins (Basel). 2020. PMID: 33302573 Free PMC article.
-
Medical Countermeasures against Ricin Intoxication.Toxins (Basel). 2023 Jan 20;15(2):100. doi: 10.3390/toxins15020100. Toxins (Basel). 2023. PMID: 36828415 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

