A Positive Feedback Loop of Hippo- and c-Jun-Amino-Terminal Kinase Signaling Pathways Regulates Amyloid-Beta-Mediated Neurodegeneration
- PMID: 32232042
- PMCID: PMC7082232
- DOI: 10.3389/fcell.2020.00117
A Positive Feedback Loop of Hippo- and c-Jun-Amino-Terminal Kinase Signaling Pathways Regulates Amyloid-Beta-Mediated Neurodegeneration
Abstract
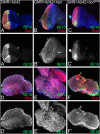
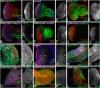
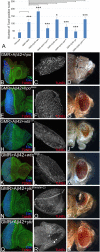
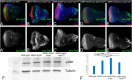
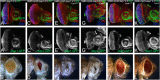
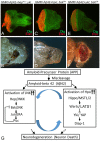
Alzheimer's disease (AD, OMIM: 104300) is an age-related disorder that affects millions of people. One of the underlying causes of AD is generation of hydrophobic amyloid-beta 42 (Aβ42) peptides that accumulate to form amyloid plaques. These plaques induce oxidative stress and aberrant signaling, which result in the death of neurons and other pathologies linked to neurodegeneration. We have developed a Drosophila eye model of AD by targeted misexpression of human Aβ42 in the differentiating retinal neurons, where an accumulation of Aβ42 triggers a characteristic neurodegenerative phenotype. In a forward deficiency screen to look for genetic modifiers, we identified a molecularly defined deficiency, which suppresses Aβ42-mediated neurodegeneration. This deficiency uncovers hippo (hpo) gene, a member of evolutionarily conserved Hippo signaling pathway that regulates growth. Activation of Hippo signaling causes cell death, whereas downregulation of Hippo signaling triggers cell proliferation. We found that Hippo signaling is activated in Aβ42-mediated neurodegeneration. Downregulation of Hippo signaling rescues the Aβ42-mediated neurodegeneration, whereas upregulation of Hippo signaling enhances the Aβ42-mediated neurodegeneration phenotypes. It is known that c-Jun-amino-terminal kinase (JNK) signaling pathway is upregulated in AD. We found that activation of JNK signaling enhances the Aβ42-mediated neurodegeneration, whereas downregulation of JNK signaling rescues the Aβ42-mediated neurodegeneration. We tested the nature of interactions between Hippo signaling and JNK signaling in Aβ42-mediated neurodegeneration using genetic epistasis approach. Our data suggest that Hippo signaling and JNK signaling, two independent signaling pathways, act synergistically upon accumulation of Aβ42 plaques to trigger cell death. Our studies demonstrate a novel role of Hippo signaling pathway in Aβ42-mediated neurodegeneration.
Keywords: Alzheimer's disease; Drosophila eye; Hippo signaling; amyloid-beta 42; c-Jun-amino-terminal kinase (JNK) signaling; cell death; growth regulation; neurodegeneration.
Copyright © 2020 Irwin, Tare, Singh, Puli, Gogia, Riccetti, Deshpande, Kango-Singh and Singh.
Figures

Similar articles
-
Inactivation of Hippo and cJun-N-terminal Kinase (JNK) signaling mitigate FUS mediated neurodegeneration in vivo.Neurobiol Dis. 2020 Jul;140:104837. doi: 10.1016/j.nbd.2020.104837. Epub 2020 Mar 19. Neurobiol Dis. 2020. PMID: 32199908 Free PMC article.
-
N-Acetyltransferase 9 ameliorates Aβ42-mediated neurodegeneration in the Drosophila eye.Cell Death Dis. 2023 Jul 28;14(7):478. doi: 10.1038/s41419-023-05973-z. Cell Death Dis. 2023. PMID: 37507384 Free PMC article.
-
A soy protein Lunasin can ameliorate amyloid-beta 42 mediated neurodegeneration in Drosophila eye.Sci Rep. 2018 Sep 10;8(1):13545. doi: 10.1038/s41598-018-31787-7. Sci Rep. 2018. PMID: 30202077 Free PMC article.
-
Alzheimer's disease: the silver tsunami of the 21(st) century.Neural Regen Res. 2016 May;11(5):693-7. doi: 10.4103/1673-5374.182680. Neural Regen Res. 2016. PMID: 27335537 Free PMC article. Review.
-
Current advances on different kinases involved in tau phosphorylation, and implications in Alzheimer's disease and tauopathies.Curr Alzheimer Res. 2005 Jan;2(1):3-18. doi: 10.2174/1567205052772713. Curr Alzheimer Res. 2005. PMID: 15977985 Review.
Cited by
-
An induced pluripotent stem cell-based model identifies molecular targets of vincristine neurotoxicity.Dis Model Mech. 2022 Dec 1;15(12):dmm049471. doi: 10.1242/dmm.049471. Epub 2022 Dec 15. Dis Model Mech. 2022. PMID: 36518084 Free PMC article.
-
Molecular Subgroup Classification in Alzheimer's Disease by Transcriptomic Profiles.J Mol Neurosci. 2022 Apr;72(4):866-879. doi: 10.1007/s12031-021-01957-w. Epub 2022 Jan 26. J Mol Neurosci. 2022. PMID: 35080766
-
Identification of Hub Genes Related to Alzheimer's Disease and Major Depressive Disorder.Am J Alzheimers Dis Other Demen. 2021 Jan-Dec;36:15333175211046123. doi: 10.1177/15333175211046123. Am J Alzheimers Dis Other Demen. 2021. PMID: 34732058 Free PMC article.
-
Extracellular microRNA and cognitive function in a prospective cohort of older men: The Veterans Affairs Normative Aging Study.Aging (Albany NY). 2022 Sep 6;14(17):6859-6886. doi: 10.18632/aging.204268. Epub 2022 Sep 6. Aging (Albany NY). 2022. PMID: 36069796 Free PMC article.
-
Hippo signaling: bridging the gap between cancer and neurodegenerative disorders.Neural Regen Res. 2021 Apr;16(4):643-652. doi: 10.4103/1673-5374.295273. Neural Regen Res. 2021. PMID: 33063715 Free PMC article.
References
-
- Battaglia C., Venturin M., Sojic A., Jesuthasan N., Orro A., Spinelli R., et al. . (2019). Candidate genes and MiRNAs linked to the inverse relationship between cancer and Alzheimer's disease: insights from data mining and enrichment analysis. Front. Genet. 10:846. 10.3389/fgene.2019.00846 - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

