Single-Cell Protein and RNA Expression Analysis of Mononuclear Phagocytes in Intestinal Mucosa and Mesenteric Lymph Nodes of Ulcerative Colitis and Crohn's Disease Patients
- PMID: 32230977
- PMCID: PMC7226791
- DOI: 10.3390/cells9040813
Single-Cell Protein and RNA Expression Analysis of Mononuclear Phagocytes in Intestinal Mucosa and Mesenteric Lymph Nodes of Ulcerative Colitis and Crohn's Disease Patients
Abstract
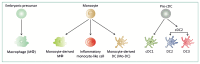
Inflammatory bowel diseases (IBDs), which include Crohn's disease (CD) and ulcerative colitis (UC), are driven by an abnormal immune response to commensal microbiota in genetically susceptible hosts. In addition to epithelial and stromal cells, innate and adaptive immune systems are both involved in IBD immunopathogenesis. Given the advances driven by single-cell technologies, we here reviewed the immune landscape and function of mononuclear phagocytes in inflamed non-lymphoid and lymphoid tissues of CD and UC patients. Immune cell profiling of IBD tissues using scRNA sequencing combined with multi-color cytometry analysis identifies unique clusters of monocyte-like cells, macrophages, and dendritic cells. These clusters reflect either distinct cell lineages (nature), or distinct or intermediate cell types with identical ontogeny, adapting their phenotype and function to the surrounding milieu (nurture and tissue imprinting). These advanced technologies will provide an unprecedented view of immune cell networks in health and disease, and thus may offer a personalized medicine approach to patients with IBD.
Keywords: Crohn’s disease; flow cytometry; inflammatory bowel disease; mononuclear phagocytes; single cell RNA sequencing; ulcerative colitis.
Conflict of interest statement
The authors have no conflict of interest to declare.
Figures




Similar articles
-
Transcriptomic Analysis and High-dimensional Phenotypic Mapping of Mononuclear Phagocytes in Mesenteric Lymph Nodes Reveal Differences Between Ulcerative Colitis and Crohn's Disease.J Crohns Colitis. 2020 Mar 13;14(3):393-405. doi: 10.1093/ecco-jcc/jjz156. J Crohns Colitis. 2020. PMID: 31541232 Free PMC article.
-
Single-Cell Analyses of Colon and Blood Reveal Distinct Immune Cell Signatures of Ulcerative Colitis and Crohn's Disease.Gastroenterology. 2020 Aug;159(2):591-608.e10. doi: 10.1053/j.gastro.2020.04.074. Epub 2020 May 16. Gastroenterology. 2020. PMID: 32428507 Free PMC article.
-
Microbial Signatures and Innate Immune Gene Expression in Lamina Propria Phagocytes of Inflammatory Bowel Disease Patients.Cell Mol Gastroenterol Hepatol. 2020;9(3):387-402. doi: 10.1016/j.jcmgh.2019.10.013. Epub 2019 Nov 15. Cell Mol Gastroenterol Hepatol. 2020. PMID: 31740421 Free PMC article.
-
Development, validation and implementation of an in vitro model for the study of metabolic and immune function in normal and inflamed human colonic epithelium.Dan Med J. 2015 Jan;62(1):B4973. Dan Med J. 2015. PMID: 25557335 Review.
-
Innate Lymphoid Cells in Intestinal Homeostasis and Inflammatory Bowel Disease.Int J Mol Sci. 2021 Jul 16;22(14):7618. doi: 10.3390/ijms22147618. Int J Mol Sci. 2021. PMID: 34299236 Free PMC article. Review.
Cited by
-
Selective oxidative protection leads to tissue topological changes orchestrated by macrophage during ulcerative colitis.Nat Commun. 2023 Jun 21;14(1):3675. doi: 10.1038/s41467-023-39173-2. Nat Commun. 2023. PMID: 37344477 Free PMC article.
-
Dendritic cells: the yin and yang in disease progression.Front Immunol. 2024 Jan 4;14:1321051. doi: 10.3389/fimmu.2023.1321051. eCollection 2023. Front Immunol. 2024. PMID: 38239364 Free PMC article. Review.
-
Single-cell RNA-sequencing combined with bulk RNA-sequencing analysis of peripheral blood reveals the characteristics and key immune cell genes of ulcerative colitis.World J Clin Cases. 2022 Nov 26;10(33):12116-12135. doi: 10.12998/wjcc.v10.i33.12116. World J Clin Cases. 2022. PMID: 36483809 Free PMC article.
-
Characterization of intestinal mononuclear phagocyte subsets in young ruminants at homeostasis and during Cryptosporidium parvum infection.Front Immunol. 2024 May 2;15:1379798. doi: 10.3389/fimmu.2024.1379798. eCollection 2024. Front Immunol. 2024. PMID: 38756777 Free PMC article.
-
Abnormal Blood Bacteriome, Gut Dysbiosis, and Progression to Severe Dengue Disease.Front Cell Infect Microbiol. 2022 Jun 17;12:890817. doi: 10.3389/fcimb.2022.890817. eCollection 2022. Front Cell Infect Microbiol. 2022. PMID: 35782108 Free PMC article.
References
-
- Watchmaker P.B., Lahl K., Lee M., Baumjohann D., Morton J., Kim S.J., Zeng R., Dent A., Ansel K.M., Diamond B., et al. Comparative transcriptional and functional profiling defines conserved programs of intestinal DC differentiation in humans and mice. Nat. Immunol. 2014;15:98–108. doi: 10.1038/ni.2768. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

