Epitope Mapping and Fine Specificity of Human T and B Cell Responses for Novel Candidate Blood-Stage Malaria Vaccine P27A
- PMID: 32210975
- PMCID: PMC7076177
- DOI: 10.3389/fimmu.2020.00412
Epitope Mapping and Fine Specificity of Human T and B Cell Responses for Novel Candidate Blood-Stage Malaria Vaccine P27A
Abstract
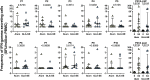
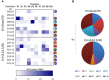
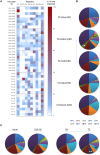
P27A is a novel synthetic malaria vaccine candidate derived from the blood stage Plasmodium falciparum protein Trophozoite Exported Protein 1 (TEX1/PFF0165c). In phase 1a/1b clinical trials in malaria unexposed adults in Switzerland and in malaria pre-exposed adults in Tanzania, P27A formulated with Alhydrogel and GLA-SE adjuvants induced antigen-specific antibodies and T-cell activity. The GLA-SE adjuvant induced significantly stronger humoral responses than the Alhydrogel adjuvant. Groups of pre-exposed and unexposed subjects received identical vaccine formulations, which supported the comparison of the cellular and humoral response to P27A in terms of fine specificity and affinity for populations and adjuvants. Globally, fine specificity of the T and B cell responses exhibited preferred recognized sequences and did not highlight major differences between adjuvants or populations. Affinity of anti-P27A antibodies was around 10-8 M in all groups. Pre-exposed volunteers presented anti-P27A with higher affinity than unexposed volunteers. Increasing the dose of GLA-SE from 2.5 to 5 μg in pre-exposed volunteers improved anti-P27A affinity and decreased the number of recognized epitopes. These results indicate a higher maturation of the humoral response in pre-exposed volunteers, particularly when immunized with P27A formulated with 5 μg GLA-SE.
Keywords: P27A; Plasmodium falciparum; adjuvant; clinical trial; immune response; malaria; populations; vaccine.
Copyright © 2020 Geiger, Guignard, Yang, Bikorimana, Correia, Houard, Mkindi, Daubenberger, Spertini, Corradin and Audran.
Figures

Similar articles
-
The Candidate Blood-stage Malaria Vaccine P27A Induces a Robust Humoral Response in a Fast Track to the Field Phase 1 Trial in Exposed and Nonexposed Volunteers.Clin Infect Dis. 2019 Jan 18;68(3):466-474. doi: 10.1093/cid/ciy514. Clin Infect Dis. 2019. PMID: 29945169 Clinical Trial.
-
Safety and immunogenicity of a recombinant Plasmodium falciparum AMA1-DiCo malaria vaccine adjuvanted with GLA-SE or Alhydrogel® in European and African adults: A phase 1a/1b, randomized, double-blind multi-centre trial.Vaccine. 2017 Oct 27;35(45):6218-6227. doi: 10.1016/j.vaccine.2017.09.027. Epub 2017 Sep 22. Vaccine. 2017. PMID: 28947345 Clinical Trial.
-
Immunogenicity of dimorphic and C-terminal fragments of Plasmodium falciparum MSP2 formulated with different adjuvants in mice.Vaccine. 2016 Mar 18;34(13):1566-1574. doi: 10.1016/j.vaccine.2016.02.013. Epub 2016 Feb 11. Vaccine. 2016. PMID: 26874325
-
T cell responses to repeat and non-repeat regions of the circumsporozoite protein detected in volunteers immunized with Plasmodium falciparum sporozoites.Mem Inst Oswaldo Cruz. 1992;87 Suppl 3:223-7. doi: 10.1590/s0074-02761992000700037. Mem Inst Oswaldo Cruz. 1992. PMID: 1364202 Review.
-
Malaria vaccine adjuvants: latest update and challenges in preclinical and clinical research.Biomed Res Int. 2013;2013:282913. doi: 10.1155/2013/282913. Epub 2013 Apr 23. Biomed Res Int. 2013. PMID: 23710439 Free PMC article. Review.
References
-
- WHO (2019). World Malaria Report 2019. Geneva.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

