Morphine exposure exacerbates HIV-1 Tat driven changes to neuroinflammatory factors in cultured astrocytes
- PMID: 32210470
- PMCID: PMC7094849
- DOI: 10.1371/journal.pone.0230563
Morphine exposure exacerbates HIV-1 Tat driven changes to neuroinflammatory factors in cultured astrocytes
Abstract
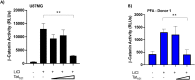
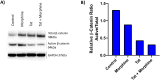
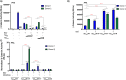
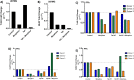
Despite antiretroviral therapy human immunodeficiency virus type-1 (HIV-1) infection results in neuroinflammation of the central nervous system that can cause HIV-associated neurocognitive disorders (HAND). The molecular mechanisms involved in the development of HAND are unclear, however, they are likely due to both direct and indirect consequences of HIV-1 infection and inflammation of the central nervous system. Additionally, opioid abuse in infected individuals has the potential to exacerbate HIV-comorbidities, such as HAND. Although restricted for productive HIV replication, astrocytes (comprising 40-70% of all brain cells) likely play a significant role in neuropathogenesis in infected individuals due to the production and response of viral proteins. The HIV-1 protein Tat is critical for viral transcription, causes neuroinflammation, and can be secreted from infected cells to affect uninfected bystander cells. The Wnt/β-catenin signaling cascade plays an integral role in restricting HIV-1 infection in part by negatively regulating HIV-1 Tat function. Conversely, Tat can overcome this negative regulation and inhibit β-catenin signaling by sequestering the critical transcription factor TCF-4 from binding to β-catenin. Here, we aimed to explore how opiate exposure affects Tat-mediated suppression of β-catenin in astrocytes and the downstream modulation of neuroinflammatory genes. We observed that morphine can potentiate Tat suppression of β-catenin activity in human astrocytes. In contrast, Tat mutants deficient in secretion, and lacking neurotoxic effects, do not affect β-catenin activity in the presence or absence of morphine. Finally, morphine treatment of astrocytes was sufficient to reduce the expression of genes involved in neuroinflammation. Examining the molecular mechanisms of how HIV-1 infection and opiate exposure exacerbate neuroinflammation may help us inform or predict disease progression prior to HAND development.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

Similar articles
-
Methamphetamine and HIV-1 Tat down regulate β-catenin signaling: implications for methampetamine abuse and HIV-1 co-morbidity.J Neuroimmune Pharmacol. 2011 Dec;6(4):597-607. doi: 10.1007/s11481-011-9295-2. Epub 2011 Jul 9. J Neuroimmune Pharmacol. 2011. PMID: 21744004 Free PMC article.
-
Human immunodeficiency virus type 1 (HIV-1) transactivator of transcription through its intact core and cysteine-rich domains inhibits Wnt/β-catenin signaling in astrocytes: relevance to HIV neuropathogenesis.J Neurosci. 2012 Nov 14;32(46):16306-13. doi: 10.1523/JNEUROSCI.3145-12.2012. J Neurosci. 2012. PMID: 23152614 Free PMC article.
-
Morphine exacerbates HIV-1 Tat-induced cytokine production in astrocytes through convergent effects on [Ca(2+)](i), NF-kappaB trafficking and transcription.PLoS One. 2008;3(12):e4093. doi: 10.1371/journal.pone.0004093. Epub 2008 Dec 31. PLoS One. 2008. PMID: 19116667 Free PMC article.
-
Role of mu-opioids as cofactors in human immunodeficiency virus type 1 disease progression and neuropathogenesis.J Neurovirol. 2011 Aug;17(4):291-302. doi: 10.1007/s13365-011-0037-2. Epub 2011 Jul 7. J Neurovirol. 2011. PMID: 21735315 Free PMC article. Review.
-
Mini review: Promotion of substance abuse in HIV patients: Biological mediation by HIV-1 Tat protein.Neurosci Lett. 2021 May 14;753:135877. doi: 10.1016/j.neulet.2021.135877. Epub 2021 Apr 7. Neurosci Lett. 2021. PMID: 33838257 Review.
Cited by
-
Drugs of Abuse and Their Impact on Viral Pathogenesis.Viruses. 2021 Nov 29;13(12):2387. doi: 10.3390/v13122387. Viruses. 2021. PMID: 34960656 Free PMC article. Review.
-
Escalating morphine dosing in HIV-1 Tat transgenic mice with sustained Tat exposure reveals an allostatic shift in neuroinflammatory regulation accompanied by increased neuroprotective non-endocannabinoid lipid signaling molecules and amino acids.J Neuroinflammation. 2020 Nov 18;17(1):345. doi: 10.1186/s12974-020-01971-6. J Neuroinflammation. 2020. PMID: 33208151 Free PMC article.
-
Opioid and neuroHIV Comorbidity - Current and Future Perspectives.J Neuroimmune Pharmacol. 2020 Dec;15(4):584-627. doi: 10.1007/s11481-020-09941-8. Epub 2020 Sep 2. J Neuroimmune Pharmacol. 2020. PMID: 32876803 Free PMC article. Review.
-
Opioid-Mediated HIV-1 Immunopathogenesis.J Neuroimmune Pharmacol. 2020 Dec;15(4):628-642. doi: 10.1007/s11481-020-09960-5. Epub 2020 Oct 8. J Neuroimmune Pharmacol. 2020. PMID: 33029670 Free PMC article. Review.
-
Priming Astrocytes With HIV-Induced Reactive Oxygen Species Enhances Their Trypanosoma cruzi Infection.Front Microbiol. 2020 Oct 19;11:563320. doi: 10.3389/fmicb.2020.563320. eCollection 2020. Front Microbiol. 2020. PMID: 33193149 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

