Acetylation of Aβ42 at Lysine 16 Disrupts Amyloid Formation
- PMID: 32207962
- PMCID: PMC7605495
- DOI: 10.1021/acschemneuro.0c00069
Acetylation of Aβ42 at Lysine 16 Disrupts Amyloid Formation
Abstract
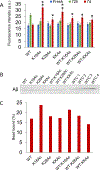
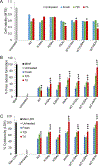
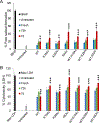
The residue lysine 28 (K28) is known to form an important salt bridge that stabilizes the Aβ amyloid structure, and acetylation of lysine 28 (K28Ac) slows the Aβ42 fibrillization rate but does not affect fibril morphology. On the other hand, acetylation of lysine 16 (K16Ac) residue greatly diminishes the fibrillization property of Aβ42 peptide and also affects its toxicity. This is due to the fact that lysine 16 acetylated amyloid beta peptide forms amorphous aggregates instead of amyloid fibrils. This is likely a result of increased hydrophobicity of the K16-A21 region due to K16 acetylation, as confirmed by molecular dynamic simulation studies. The calculated results show that the hydrophobic patches of aggregates from acetylated peptides were different when compared to wild-type (WT) peptide. K16Ac and double acetylated (KKAc) peptide aggregates show significantly higher cytotoxicity compared to the WT or K28Ac peptide aggregates alone. However, the heterogeneous mixture of WT and acetylated Aβ42 peptide aggregates exhibited higher free radical formation as well as cytotoxicity, suggesting dynamic interactions between different species could be a critical contributor to Aβ pathology.
Keywords: acetylation; aggregation; amyloid fibril; amyloid β peptide; molecular dynamics; post-translational modifications; toxicity.
Conflict of interest statement
The authors declare no competing financial interest.
Figures



Similar articles
-
Acetylation of Aβ40 Alters Aggregation in the Presence and Absence of Lipid Membranes.ACS Chem Neurosci. 2020 Jan 15;11(2):146-161. doi: 10.1021/acschemneuro.9b00483. Epub 2019 Dec 27. ACS Chem Neurosci. 2020. PMID: 31834770 Free PMC article.
-
Site-specific effects of peptide lipidation on beta-amyloid aggregation and cytotoxicity.J Biol Chem. 2007 Dec 21;282(51):36987-97. doi: 10.1074/jbc.M702146200. Epub 2007 Aug 9. J Biol Chem. 2007. PMID: 17693400
-
Influence of preformed Asp23-Lys28 salt bridge on the conformational fluctuations of monomers and dimers of Abeta peptides with implications for rates of fibril formation.J Phys Chem B. 2009 Jan 29;113(4):1162-72. doi: 10.1021/jp808914c. J Phys Chem B. 2009. PMID: 19125574 Free PMC article.
-
Lysine Acetylation Changes the Mechanism of Aβ25-35 Peptide Binding and Dimerization in the DMPC Bilayer.ACS Chem Neurosci. 2023 Feb 1;14(3):494-505. doi: 10.1021/acschemneuro.2c00722. Epub 2023 Jan 19. ACS Chem Neurosci. 2023. PMID: 36656569
-
Understanding amyloid fibril nucleation and aβ oligomer/drug interactions from computer simulations.Acc Chem Res. 2014 Feb 18;47(2):603-11. doi: 10.1021/ar4002075. Epub 2013 Dec 24. Acc Chem Res. 2014. PMID: 24368046 Review.
Cited by
-
Deciphering the Effect of Lysine Acetylation on the Misfolding and Aggregation of Human Tau Fragment 171IPAKTPPAPK180 Using Molecular Dynamic Simulation and the Markov State Model.Int J Mol Sci. 2022 Feb 22;23(5):2399. doi: 10.3390/ijms23052399. Int J Mol Sci. 2022. PMID: 35269542 Free PMC article.
-
The role of altered protein acetylation in neurodegenerative disease.Front Aging Neurosci. 2023 Jan 4;14:1025473. doi: 10.3389/fnagi.2022.1025473. eCollection 2022. Front Aging Neurosci. 2023. PMID: 36688174 Free PMC article. Review.
-
A Chemical Mutagenesis Approach to Insert Post-translational Modifications in Aggregation-Prone Proteins.ACS Chem Neurosci. 2022 Jun 15;13(12):1714-1718. doi: 10.1021/acschemneuro.2c00077. Epub 2022 May 24. ACS Chem Neurosci. 2022. PMID: 35609278 Free PMC article.
-
Phase separation and pathologic transitions of RNP condensates in neurons: implications for amyotrophic lateral sclerosis, frontotemporal dementia and other neurodegenerative disorders.Front Mol Neurosci. 2023 Sep 1;16:1242925. doi: 10.3389/fnmol.2023.1242925. eCollection 2023. Front Mol Neurosci. 2023. PMID: 37720552 Free PMC article. Review.
-
Abnormal protein post-translational modifications induces aggregation and abnormal deposition of protein, mediating neurodegenerative diseases.Cell Biosci. 2024 Feb 12;14(1):22. doi: 10.1186/s13578-023-01189-y. Cell Biosci. 2024. PMID: 38347638 Free PMC article. Review.
References
-
- Assoc A (2015) Alzheimer’s Association Report 2015 Alzheimer’s disease facts and figures. Alzheimer’s Dementia 11 (3), 332–384. - PubMed
-
- Ballard C, Gauthier S, Corbett A, Brayne C, Aarsland D, and Jones E (2011) Alzheimer’s disease. Lancet 377 (9770), 1019–1031. - PubMed
-
- Atwood CS, Obrenovich ME, Liu TB, Chan H, Perry G, Smith MA, and Martins RN (2003) Amyloid-beta: a chameleon walking in two worlds: a review of the trophic and toxic properties of amyloid-beta. Brain Res. Rev 43 (1), 1–16. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

