How I treat CMV reactivation after allogeneic hematopoietic stem cell transplantation
- PMID: 32202631
- PMCID: PMC7484743
- DOI: 10.1182/blood.2019000956
How I treat CMV reactivation after allogeneic hematopoietic stem cell transplantation
Abstract
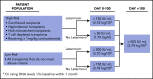
Cytomegalovirus (CMV) reactivation remains one of the most common and life-threatening infectious complications following allogeneic hematopoietic stem cell transplantation, despite novel diagnostic technologies, several novel prophylactic agents, and further improvements in preemptive therapy and treatment of established CMV disease. Treatment decisions for CMV reactivation are becoming increasingly difficult and must take into account whether the patient has received antiviral prophylaxis, the patient's individual risk profile for CMV disease, CMV-specific T-cell reconstitution, CMV viral load, and the potential drug resistance detected at the time of initiation of antiviral therapy. Thus, we increasingly use personalized treatment strategies for the recipient of an allograft with CMV reactivation based on prior use of anti-CMV prophylaxis, viral load, the assessment of CMV-specific T-cell immunity, and the molecular assessment of resistance to antiviral drugs.
© 2020 by The American Society of Hematology.
Conflict of interest statement
Conflict-of-interest disclosure: P.L. reports personal fees from AiCuris and grants from Astellas, Oxford Immunotech, Takeda (Shire), and MSD outside of the submitted work. M.B. reports grants and personal fees from Merck, Takeda/Shire, Gilead Sciences, and VirBio; grants from Astellas and Chimerix; personal fees, including options to acquire equity from Helocyte and EvrysBio; and personal fees from GlaxoSmithKline, Moderna, and AlloVir, outside of the submitted work. H.E. declares no competing financial interests.
Figures


Similar articles
-
Letermovir for primary and secondary cytomegalovirus prevention in allogeneic hematopoietic cell transplant recipients: Real-world experience.Transpl Infect Dis. 2019 Dec;21(6):e13187. doi: 10.1111/tid.13187. Epub 2019 Oct 21. Transpl Infect Dis. 2019. PMID: 31585500 Free PMC article.
-
Fast breakthrough of resistant cytomegalovirus during secondary letermovir prophylaxis in a hematopoietic stem cell transplant recipient.BMC Infect Dis. 2019 May 8;19(1):388. doi: 10.1186/s12879-019-4016-1. BMC Infect Dis. 2019. PMID: 31068147 Free PMC article.
-
Pre-transplant cytomegalovirus (CMV) serostatus remains the most important determinant of CMV reactivation after allogeneic hematopoietic stem cell transplantation in the era of surveillance and preemptive therapy.Transpl Infect Dis. 2010 Aug 1;12(4):322-9. doi: 10.1111/j.1399-3062.2010.00504.x. Epub 2010 May 11. Transpl Infect Dis. 2010. PMID: 20487414
-
[Cytomegalovirus infections following allogeneic hematopoietic stem cell transplantation: prophylaxis and treatment].Rinsho Ketsueki. 2019;60(6):635-645. doi: 10.11406/rinketsu.60.635. Rinsho Ketsueki. 2019. PMID: 31281156 Review. Japanese.
-
[Management of cytomegalovirus infection after hematopoietic stem cell transplantation].Rinsho Ketsueki. 2019;60(9):1337-1340. doi: 10.11406/rinketsu.60.1337. Rinsho Ketsueki. 2019. PMID: 31597861 Review. Japanese.
Cited by
-
Posttransplant cyclophosphamide is associated with increased cytomegalovirus infection: a CIBMTR analysis.Blood. 2021 Jun 10;137(23):3291-3305. doi: 10.1182/blood.2020009362. Blood. 2021. PMID: 33657221 Free PMC article. Clinical Trial.
-
Treatment for First Cytomegalovirus Infection Post-Hematopoietic Cell Transplant in the AURORA Trial: A Multicenter, Double-Blind, Randomized, Phase 3 Trial Comparing Maribavir With Valganciclovir.Clin Infect Dis. 2024 Mar 20;78(3):562-572. doi: 10.1093/cid/ciad709. Clin Infect Dis. 2024. PMID: 38036487 Free PMC article. Clinical Trial.
-
Pre-engraftment Cytomegalovirus DNAemia after allogeneic hematopoietic stem cell transplantation and its impact on engraftment.Bone Marrow Transplant. 2022 Feb;57(2):289-291. doi: 10.1038/s41409-021-01520-6. Epub 2021 Nov 1. Bone Marrow Transplant. 2022. PMID: 34725477 No abstract available.
-
"Cerberus" T Cells: A Glucocorticoid-Resistant, Multi-Pathogen Specific T Cell Product to Fight Infections in Severely Immunocompromised Patients.Front Immunol. 2021 Jan 18;11:608701. doi: 10.3389/fimmu.2020.608701. eCollection 2020. Front Immunol. 2021. PMID: 33537032 Free PMC article.
-
Differential clinical impact of letermovir prophylaxis according to graft sources: a KSGCT multicenter retrospective analysis.Blood Adv. 2024 Mar 12;8(5):1084-1093. doi: 10.1182/bloodadvances.2023010735. Blood Adv. 2024. PMID: 38330190 Free PMC article.
References
-
- Zaia JA, Gallez-Hawkins GM, Tegtmeier BR, et al. . Late cytomegalovirus disease in marrow transplantation is predicted by virus load in plasma. J Infect Dis. 1997;176(3):782-785. - PubMed
-
- Fuji S, Einsele H, Kapp M. Cytomegalovirus disease in hematopoietic stem cell transplant patients: current and future therapeutic options. Curr Opin Infect Dis. 2017;30(4):372-376. - PubMed
-
- Chemaly RF, Chou S, Einsele H, et al. ; Resistant Definitions Working Group of the Cytomegalovirus Drug Development Forum . Definitions of resistant and refractory cytomegalovirus infection and disease in transplant recipients for use in clinical trials. Clin Infect Dis. 2019;68(8):1420-1426. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

