NK cells mediate clearance of CD8+ T cell-resistant tumors in response to STING agonists
- PMID: 32198222
- PMCID: PMC7228660
- DOI: 10.1126/sciimmunol.aaz2738
NK cells mediate clearance of CD8+ T cell-resistant tumors in response to STING agonists
Abstract
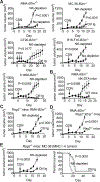
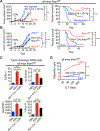
Several immunotherapy approaches that mobilize CD8+ T cell responses stimulate tumor rejection, and some, such as checkpoint blockade, have been approved for several cancer indications and show impressive increases in patient survival. However, tumors may evade CD8+ T cell recognition via loss of MHC molecules or because they contain few or no neoantigens. Therefore, approaches are needed to combat CD8+ T cell-resistant cancers. STING-activating cyclic dinucleotides (CDNs) are a new class of immune-stimulating agents that elicit impressive CD8+ T cell-mediated tumor rejection in preclinical tumor models and are now being tested in clinical trials. Here, we demonstrate powerful CDN-induced, natural killer (NK) cell-mediated tumor rejection in numerous tumor models, independent of CD8+ T cells. CDNs enhanced NK cell activation, cytotoxicity, and antitumor effects in part by inducing type I interferon (IFN). IFN acted in part directly on NK cells in vivo and in part indirectly via the induction of IL-15 and IL-15 receptors, which were important for CDN-induced NK activation and tumor control. After in vivo administration of CDNs, dendritic cells (DCs) up-regulated IL-15Rα in an IFN-dependent manner. Mice lacking the type I IFN receptor specifically on DCs had reduced NK cell activation and tumor control. Therapeutics that activate NK cells, such as CDNs, checkpoint inhibitors, NK cell engagers, and cytokines, may represent next-generation approaches to cancer immunotherapy.
Copyright © 2020 The Authors, some rights reserved; exclusive licensee American Association for the Advancement of Science. No claim to original U.S. Government Works.
Figures

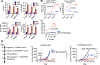



Similar articles
-
Manganese is critical for antitumor immune responses via cGAS-STING and improves the efficacy of clinical immunotherapy.Cell Res. 2020 Nov;30(11):966-979. doi: 10.1038/s41422-020-00395-4. Epub 2020 Aug 24. Cell Res. 2020. PMID: 32839553 Free PMC article. Clinical Trial.
-
Synergy of a STING agonist and an IL-2 superkine in cancer immunotherapy against MHC I-deficient and MHC I+ tumors.Proc Natl Acad Sci U S A. 2022 May 31;119(22):e2200568119. doi: 10.1073/pnas.2200568119. Epub 2022 May 19. Proc Natl Acad Sci U S A. 2022. PMID: 35588144 Free PMC article.
-
NK-cell activation by LIGHT triggers tumor-specific CD8+ T-cell immunity to reject established tumors.Blood. 2006 Feb 15;107(4):1342-51. doi: 10.1182/blood-2005-08-3485. Epub 2005 Oct 13. Blood. 2006. PMID: 16223768 Free PMC article.
-
Enhancing Natural Killer and CD8+ T Cell-Mediated Anticancer Cytotoxicity and Proliferation of CD8+ T Cells with HLA-E Monospecific Monoclonal Antibodies.Monoclon Antib Immunodiagn Immunother. 2019 Apr;38(2):38-59. doi: 10.1089/mab.2018.0043. Monoclon Antib Immunodiagn Immunother. 2019. PMID: 31009335 Free PMC article. Review.
-
Natural killer-dendritic cell cross-talk in cancer immunotherapy.Expert Opin Biol Ther. 2005 Oct;5(10):1303-15. doi: 10.1517/14712598.5.10.1303. Expert Opin Biol Ther. 2005. PMID: 16197336 Review.
Cited by
-
Natural Killer Cells: The Linchpin for Successful Cancer Immunotherapy.Front Immunol. 2021 Apr 28;12:679117. doi: 10.3389/fimmu.2021.679117. eCollection 2021. Front Immunol. 2021. PMID: 33995422 Free PMC article. Review.
-
Harnessing natural killer cells for cancer immunotherapy: dispatching the first responders.Nat Rev Drug Discov. 2022 Aug;21(8):559-577. doi: 10.1038/s41573-022-00413-7. Epub 2022 Mar 21. Nat Rev Drug Discov. 2022. PMID: 35314852 Free PMC article. Review.
-
Gut microbiota influence immunotherapy responses: mechanisms and therapeutic strategies.J Hematol Oncol. 2022 Apr 29;15(1):47. doi: 10.1186/s13045-022-01273-9. J Hematol Oncol. 2022. PMID: 35488243 Free PMC article. Review.
-
Leveraging NKG2D Ligands in Immuno-Oncology.Front Immunol. 2021 Jul 29;12:713158. doi: 10.3389/fimmu.2021.713158. eCollection 2021. Front Immunol. 2021. PMID: 34394116 Free PMC article. Review.
-
Intratumoral delivery of the chitin-derived C100 adjuvant promotes robust STING, IFNAR, and CD8+ T cell-dependent anti-tumor immunity.Cell Rep Med. 2024 May 21;5(5):101560. doi: 10.1016/j.xcrm.2024.101560. Epub 2024 May 9. Cell Rep Med. 2024. PMID: 38729159 Free PMC article.
References
-
- Sharma P, Allison JP, The future of immune checkpoint therapy. Science 348, 56–61 (2015). - PubMed
-
- Hirano F, Kaneko K, Tamura H, Dong H, Wang S, Ichikawa M, Rietz C, Flies DB, Lau JS, Zhu G, Tamada K, Chen L, Blockade of B7-H1 and PD-1 by monoclonal antibodies potentiates cancer therapeutic immunity. Cancer Res 65, 1089–1096 (2005). - PubMed
-
- Leach DR, Krummel MF, Allison JP, Enhancement of antitumor immunity by CTLA-4 blockade. Science 271, 1734–1736 (1996). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

