Bioinspired tumor-homing nanoplatform for co-delivery of paclitaxel and siRNA-E7 to HPV-related cervical malignancies for synergistic therapy
- PMID: 32194871
- PMCID: PMC7053183
- DOI: 10.7150/thno.41228
Bioinspired tumor-homing nanoplatform for co-delivery of paclitaxel and siRNA-E7 to HPV-related cervical malignancies for synergistic therapy
Abstract
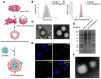
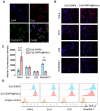
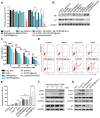
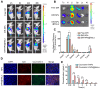
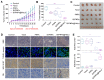
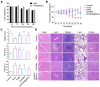
Because of the complexity of cancer, a combination of chemotherapy and gene therapy is an emerging treatment modality. To realize the full potential of this strategy, a smart, highly biocompatible nanosystem that enables the precise co-delivery of small-molecule anticancer drugs and small interfering RNA (siRNA) is urgently needed. This study aimed to improve the therapeutic effect against cervical cancer by using cancer cell membrane-camouflaged nanoparticles for simultaneous delivery of paclitaxel (PTX) and siRNA targeting E7. Methods: By camouflaging HeLa cell membranes onto siRNA/PTX co-loaded (lactic-co-glycolic acid) (PLGA) nanoparticles, a biomimetic dual-drug delivery system (Si/PNPs@HeLa) was developed to simultaneously deliver PTX and siRNA targeting E7. After evaluating the physicochemical characteristics as well as their cell uptake and biodistribution behavior, studies on the RNA interference efficiency and antitumor ability of Si/PNPs@HeLa in vitro and in vivo were further carried out. Results: The Si/PNPs@HeLa was capable of delivering PTX and siRNA simultaneously to HeLa cells both in vitro and in vivo. Moreover, benefiting from the recognition and adhesion molecules on the surface of HeLa cells, Si/PNPs@HeLa exhibited an improved immune escape ability and an increased tumor region accumulation (3-fold higher than bare nanoparticles). As a result, an excellent synergistic anti-tumor effect was observed in the HeLa tumor-bearing mice, with tumor volume inhibiting rates of 83.6% and no side effects in major organs. The mechanistic studies confirmed that E7 knockdown sensitized HeLa cells to PTX chemotherapy, mainly by inhibiting PTX-induced AKT pathway activation. Conclusion: Si/PNPs@HeLa, by integrating immune escape and tumor-homing ability, can serve as an efficient dual-drug delivery system to achieve precise treatment of cervical cancer through chemo-gene combined therapy.
Keywords: cancer cell membrane camouflage; cervical cancer; homotypic targeting; paclitaxel; siRNA-E7.
© The author(s).
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures

Similar articles
-
Co-delivery of paclitaxel and anti-VEGF siRNA by tripeptide lipid nanoparticle to enhance the anti-tumor activity for lung cancer therapy.Drug Deliv. 2020 Dec;27(1):1397-1411. doi: 10.1080/10717544.2020.1827085. Drug Deliv. 2020. PMID: 33096948 Free PMC article.
-
Stimuli-responsive magnetic silica-poly-lactic-co-glycolic acid hybrid nanoparticles for targeted cancer chemo-immunotherapy.Drug Deliv Transl Res. 2024 Oct;14(10):2712-2726. doi: 10.1007/s13346-024-01521-0. Epub 2024 Feb 12. Drug Deliv Transl Res. 2024. PMID: 38347431
-
Smart polymeric nanoparticles with pH-responsive and PEG-detachable properties for co-delivering paclitaxel and survivin siRNA to enhance antitumor outcomes.Int J Nanomedicine. 2018 Apr 20;13:2405-2426. doi: 10.2147/IJN.S161426. eCollection 2018. Int J Nanomedicine. 2018. PMID: 29719390 Free PMC article.
-
Enabling anticancer therapeutics by nanoparticle carriers: the delivery of Paclitaxel.Int J Mol Sci. 2011;12(7):4395-413. doi: 10.3390/ijms12074395. Epub 2011 Jul 7. Int J Mol Sci. 2011. PMID: 21845085 Free PMC article. Review.
-
Recent advances in delivery of drug-nucleic acid combinations for cancer treatment.J Control Release. 2013 Dec 10;172(2):589-600. doi: 10.1016/j.jconrel.2013.04.010. Epub 2013 Apr 25. J Control Release. 2013. PMID: 23624358 Free PMC article. Review.
Cited by
-
Cell Membrane-Camouflaged Nanoparticles Mediated Nucleic Acids Delivery.Int J Nanomedicine. 2023 Dec 28;18:8001-8021. doi: 10.2147/IJN.S433737. eCollection 2023. Int J Nanomedicine. 2023. PMID: 38164266 Free PMC article. Review.
-
Research Status and Prospect of Non-Viral Vectors Based on siRNA: A Review.Int J Mol Sci. 2023 Feb 8;24(4):3375. doi: 10.3390/ijms24043375. Int J Mol Sci. 2023. PMID: 36834783 Free PMC article. Review.
-
Toll-like Receptor Signaling-deficient Cells Enhance Antitumor Activity of Cell-based Immunotherapy by Increasing Tumor Homing.Cancer Res Commun. 2023 Mar 1;3(3):347-360. doi: 10.1158/2767-9764.CRC-22-0365. eCollection 2023 Mar. Cancer Res Commun. 2023. PMID: 36875156 Free PMC article.
-
Tumor-Derived Membrane Vesicles: A Promising Tool for Personalized Immunotherapy.Pharmaceuticals (Basel). 2022 Jul 16;15(7):876. doi: 10.3390/ph15070876. Pharmaceuticals (Basel). 2022. PMID: 35890175 Free PMC article. Review.
-
Recent Advances in Nanoparticle-Based Co-Delivery Systems for Cancer Therapy.Nanomaterials (Basel). 2022 Aug 4;12(15):2672. doi: 10.3390/nano12152672. Nanomaterials (Basel). 2022. PMID: 35957103 Free PMC article. Review.
References
-
- Cohen PA, Jhingran A, Oaknin A, Denny L. Cervical cancer. Lancet. 2019;393:169–82. - PubMed
-
- Moore KN, Herzog TJ, Lewin S, Giuntoli RL, Armstrong DK, Rocconi RP. et al. A comparison of cisplatin/paclitaxel and carboplatin/paclitaxel in stage IVB, recurrent or persistent cervical cancer. Gynecol Oncol. 2007;105:299–303. - PubMed
-
- Kudelka AP, Winn R, Edwards CL, Downey G, Greenberg H, Dakhil SR. et al. An update of a phase II study of paclitaxel in advanced or recurrent squamous cell cancer of the cervix. Anticancer Drugs. 1997;8:657–61. - PubMed
-
- Kitagawa R, Katsumata N, Shibata T, Kamura T, Kasamatsu T, Nakanishi T. et al. Paclitaxel plus carboplatin versus paclitaxel plus cisplatin in metastatic or recurrent cervical cancer: the open-label randomized phase III trial JCOG0505. J Clin Oncol. 2015;33:2129–35. - PubMed
-
- Saraswathy M, Gong S. Recent developments in the co-delivery of siRNA and small molecule anticancer drugs for cancer treatment. Mater Today (Kidlington) 2014;17:298–306.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

