Treatment of chronic neuropathic pain: purine receptor modulation
- PMID: 32187120
- PMCID: PMC7305993
- DOI: 10.1097/j.pain.0000000000001857
Treatment of chronic neuropathic pain: purine receptor modulation
Abstract
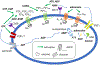
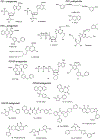
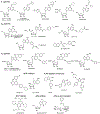
Extracellular nucleosides and nucleotides have widespread functions in responding to physiological stress. The "purinome" encompasses 4 G-protein-coupled receptors (GPCRs) for adenosine, 8 GPCRs activated by nucleotides, 7 adenosine 5'-triphosphate-gated P2X ion channels, as well as the associated enzymes and transporters that regulate native agonist levels. Purinergic signaling modulators, such as receptor agonists and antagonists, have potential for treating chronic pain. Adenosine and its analogues potently suppress nociception in preclinical models by activating A1 and/or A3 adenosine receptors (ARs), but safely harnessing this pathway to clinically treat pain has not been achieved. Both A2AAR agonists and antagonists are efficacious in pain models. Highly selective A3AR agonists offer a novel approach to treat chronic pain. We have explored the structure activity relationship of nucleoside derivatives at this subtype using a computational structure-based approach. Novel A3AR agonists for pain control containing a bicyclic ring system (bicyclo [3.1.0] hexane) in place of ribose were designed and screened using an in vivo phenotypic model, which reflected both pharmacokinetic and pharmacodynamic parameters. High specificity (>10,000-fold selective for A3AR) was achieved with the aid of receptor homology models based on related GPCR structures. These A3AR agonists are well tolerated in vivo and highly efficacious in models of chronic neuropathic pain. Furthermore, signaling molecules acting at P2X3, P2X4, P2X7, and P2Y12Rs play critical roles in maladaptive pain neuroplasticity, and their antagonists reduce chronic or inflammatory pain, and, therefore, purine receptor modulation is a promising approach for future pain therapeutics. Structurally novel antagonists for these nucleotide receptors were discovered recently.
Conflict of interest statement
Author Disclosure Statements:
Dr. Salvemini is founder of BioIntervene, Inc. a company developing A3AR agonists as analgesics for chronic pain. All other authors declare no conflict of interest.
Figures


Similar articles
-
Purinergic Signaling: Impact of GPCR Structures on Rational Drug Design.ChemMedChem. 2020 Nov 4;15(21):1958-1973. doi: 10.1002/cmdc.202000465. Epub 2020 Sep 18. ChemMedChem. 2020. PMID: 32803849 Free PMC article. Review.
-
Medicinal chemistry of adenosine, P2Y and P2X receptors.Neuropharmacology. 2016 May;104:31-49. doi: 10.1016/j.neuropharm.2015.12.001. Epub 2015 Dec 12. Neuropharmacology. 2016. PMID: 26686393 Free PMC article. Review.
-
Rational design of sulfonated A3 adenosine receptor-selective nucleosides as pharmacological tools to study chronic neuropathic pain.J Med Chem. 2013 Jul 25;56(14):5949-63. doi: 10.1021/jm4007966. Epub 2013 Jul 3. J Med Chem. 2013. PMID: 23789857 Free PMC article.
-
Agonists and Antagonists for Purinergic Receptors.Methods Mol Biol. 2020;2041:45-64. doi: 10.1007/978-1-4939-9717-6_3. Methods Mol Biol. 2020. PMID: 31646479
-
Ocular Purine Receptors as Drug Targets in the Eye.J Ocul Pharmacol Ther. 2016 Oct;32(8):534-547. doi: 10.1089/jop.2016.0090. Epub 2016 Aug 30. J Ocul Pharmacol Ther. 2016. PMID: 27574786 Free PMC article. Review.
Cited by
-
Neuroprotective Roles of the Adenosine A3 Receptor Agonist AST-004 in Mouse Model of Traumatic Brain Injury.Neurotherapeutics. 2021 Oct;18(4):2707-2721. doi: 10.1007/s13311-021-01113-7. Epub 2021 Oct 4. Neurotherapeutics. 2021. PMID: 34608616 Free PMC article.
-
Medicinal chemistry of P2 and adenosine receptors: Common scaffolds adapted for multiple targets.Biochem Pharmacol. 2021 May;187:114311. doi: 10.1016/j.bcp.2020.114311. Epub 2020 Oct 29. Biochem Pharmacol. 2021. PMID: 33130128 Free PMC article. Review.
-
Enhancing spinal cord stimulation-induced pain inhibition by augmenting endogenous adenosine signalling after nerve injury in rats.Br J Anaesth. 2024 Apr;132(4):746-757. doi: 10.1016/j.bja.2024.01.005. Epub 2024 Feb 2. Br J Anaesth. 2024. PMID: 38310069
-
Adenosine A1R/A3R agonist AST-004 reduces brain infarction in mouse and rat models of acute ischemic stroke.Front Stroke. 2022;1:1010928. doi: 10.3389/fstro.2022.1010928. Epub 2022 Nov 22. Front Stroke. 2022. PMID: 38348128 Free PMC article.
-
Purinergic Signaling: Impact of GPCR Structures on Rational Drug Design.ChemMedChem. 2020 Nov 4;15(21):1958-1973. doi: 10.1002/cmdc.202000465. Epub 2020 Sep 18. ChemMedChem. 2020. PMID: 32803849 Free PMC article. Review.
References
-
- A Phase 1-2 Study of CF102 in Patients With Advanced Hepatocellular Carcinoma. https://ClinicalTrials.gov/show/NCT00790218.
-
- Phase 2, Randomized, Double-Blind, Placebo-Controlled of the Efficacy and Safety of CF102 in Hepatocellular Carcinoma (HCC). https://ClinicalTrials.gov/show/NCT02128958.
-
- Abbracchio MP, Rainaldi G, Giammarioli AM, Ceruti S, Brambilla R, Cattabeni F, Barbieri D, Franceschi C, Jacobson KA,Malorni W. The A3 adenosine receptor mediates cell spreading, reorganization of actin cytoskeleton, and distribution of Bcl-XL: studies in human astroglioma cells. Biochem Biophys Res Commun, 1997. 241(2): p. 297–304. - PMC - PubMed
-
- Abo-Salem OM, Hayallah AM, Bilkei-Gorzo A, Filipek B, Zimmer A,Muller CE. Antinociceptive effects of novel A2B adenosine receptor antagonists. J Pharmacol Exp Ther, 2004. 308(1): p. 358–66. - PubMed
-
- Antonioli L, Fornai M, Blandizzi C, Pacher P,Hasko G. Adenosine signaling and the immune system: When a lot could be too much. Immunol Lett, 2019. 205: p. 9–15. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

