Localized cocktail chemoimmunotherapy after in situ gelation to trigger robust systemic antitumor immune responses
- PMID: 32181368
- PMCID: PMC7056299
- DOI: 10.1126/sciadv.aaz4204
Localized cocktail chemoimmunotherapy after in situ gelation to trigger robust systemic antitumor immune responses
Abstract
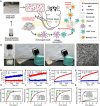
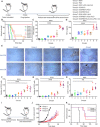
Currently, there is a huge demand to develop chemoimmunotherapy with reduced systemic toxicity and potent efficacy to combat late-stage cancers with spreading metastases. Here, we report several "cocktail" therapeutic formulations by mixing immunogenic cell death (ICD)-inducing chemotherapeutics and immune adjuvants together with alginate (ALG) for localized chemoimmunotherapy. Immune checkpoint blockade (ICB) antibody may be either included into this cocktail for local injection or used via conventional intravenous injection. After injection of such cocktail into a solid tumor, in-situ gelation of ALG would lead to local retention and sustained release of therapeutics to reduce systemic toxicity. The chemotherapy-induced ICD with the help of immune adjuvant would trigger tumor-specific immune responses, which are further amplified by ICB to elicit potent systemic antitumor immune responses in destructing local tumors, eliminating metastases and inhibiting cancer recurrence. Our strategy of combining clinically used agents for tumor-localized cocktail chemoimmunotherapy possesses great potential for clinical translation.
Copyright © 2020 The Authors, some rights reserved; exclusive licensee American Association for the Advancement of Science. No claim to original U.S. Government Works. Distributed under a Creative Commons Attribution NonCommercial License 4.0 (CC BY-NC).
Figures




Similar articles
-
Immunogenic Cell Death Amplified by Co-localized Adjuvant Delivery for Cancer Immunotherapy.Nano Lett. 2017 Dec 13;17(12):7387-7393. doi: 10.1021/acs.nanolett.7b03218. Epub 2017 Nov 22. Nano Lett. 2017. PMID: 29144754 Free PMC article.
-
Three steps to breaking immune tolerance to lymphoma: a microparticle approach.Cancer Immunol Res. 2015 Apr;3(4):389-98. doi: 10.1158/2326-6066.CIR-14-0173. Epub 2015 Jan 27. Cancer Immunol Res. 2015. PMID: 25627654 Free PMC article.
-
IL-6 and PD-L1 blockade combination inhibits hepatocellular carcinoma cancer development in mouse model.Biochem Biophys Res Commun. 2017 Apr 29;486(2):239-244. doi: 10.1016/j.bbrc.2017.02.128. Epub 2017 Mar 1. Biochem Biophys Res Commun. 2017. PMID: 28254435
-
Tumor Microenvironment-Activatable Prodrug Vesicles for Nanoenabled Cancer Chemoimmunotherapy Combining Immunogenic Cell Death Induction and CD47 Blockade.Adv Mater. 2019 Apr;31(14):e1805888. doi: 10.1002/adma.201805888. Epub 2019 Feb 14. Adv Mater. 2019. PMID: 30762908 Review.
-
Firing Up Cold Tumors.Trends Cancer. 2019 Sep;5(9):528-530. doi: 10.1016/j.trecan.2019.06.005. Epub 2019 Jul 13. Trends Cancer. 2019. PMID: 31474357 Review.
Cited by
-
Enhancing in situ cancer vaccines using delivery technologies.Nat Rev Drug Discov. 2024 Aug;23(8):607-625. doi: 10.1038/s41573-024-00974-9. Epub 2024 Jul 1. Nat Rev Drug Discov. 2024. PMID: 38951662 Review.
-
Interfacing Biomaterials with Synthetic T Cell Immunity.Adv Healthc Mater. 2021 Aug;10(15):e2100157. doi: 10.1002/adhm.202100157. Epub 2021 Apr 22. Adv Healthc Mater. 2021. PMID: 33887123 Free PMC article. Review.
-
An injectable signal-amplifying device elicits a specific immune response against malignant glioblastoma.Acta Pharm Sin B. 2023 Dec;13(12):5091-5106. doi: 10.1016/j.apsb.2023.06.010. Epub 2023 Jun 20. Acta Pharm Sin B. 2023. PMID: 38045037 Free PMC article.
-
Ultrasound (US)-activated redox dyshomeostasis therapy reinforced by immunogenic cell death (ICD) through a mitochondrial targeting liposomal nanosystem.Theranostics. 2021 Sep 13;11(19):9470-9491. doi: 10.7150/thno.62984. eCollection 2021. Theranostics. 2021. PMID: 34646381 Free PMC article.
-
Nano-immunotherapy for each stage of cancer cellular immunity: which, why, and what?Theranostics. 2021 Jun 1;11(15):7471-7487. doi: 10.7150/thno.59953. eCollection 2021. Theranostics. 2021. PMID: 34158861 Free PMC article. Review.
References
-
- Leach D. R., Krummel M. F., Allison J. P., Enhancement of antitumor immunity by CTLA-4 blockade. Science 271, 1734–1736 (1996). - PubMed
-
- Hodi F. S., O’Day S. J., McDermott D. F., Weber R. W., Sosman J. A., Haanen J. B., Gonzalez R., Robert C., Schadendorf D., Hassel J. C., Akerley W., van den Eertwegh A. J., Lutzky J., Lorigan P., Vaubel J. M., Linette G. P., Hogg D., Ottensmeier C. H., Lebbé C., Peschel C., Quirt I., Clark J. I., Wolchok J. D., Weber J. S., Tian J., Yellin M. J., Nichol G. M., Hoos A., Urba W. J., Improved survival with ipilimumab in patients with metastatic melanoma. New Eng. J. Med. 363, 711–723 (2010). - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

