PRDM3 attenuates pancreatitis and pancreatic tumorigenesis by regulating inflammatory response
- PMID: 32179733
- PMCID: PMC7075911
- DOI: 10.1038/s41419-020-2371-x
PRDM3 attenuates pancreatitis and pancreatic tumorigenesis by regulating inflammatory response
Abstract
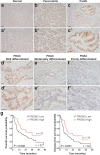
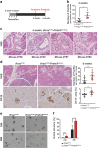
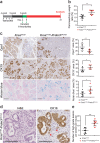
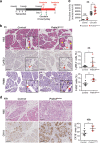
Pancreatic ductal adenocarcinoma (PDAC) is associated with metaplastic changes in the pancreas but the transcriptional program underlying these changes is incompletely understood. The zinc finger transcription factor, PRDM3, is lowly expressed in normal pancreatic acini and its expression increases during tumorigenesis. Although PRDM3 promotes proliferation and migration of PDAC cell lines, the role of PRDM3 during tumor initiation from pancreatic acinar cells in vivo is unclear. In this study, we showed that high levels of PRDM3 expression in human pancreas was associated with pancreatitis, and well-differentiated but not poorly differentiated carcinoma. We examined PRDM3 function in pancreatic acinar cells during tumor formation and pancreatitis by inactivating Prdm3 using a conditional allele (Ptf1aCreER;Prdm3flox/flox mice) in the context of oncogenic Kras expression and supraphysiological cerulein injections, respectively. In Prdm3-deficient mice, KrasG12D-driven preneoplastic lesions were more abundant and progressed to high-grade precancerous lesions more rapidly. This is consistent with our observations that low levels of PRDM3 in human PDAC was correlated significantly with poorer survival in patient. Moreover, loss of Prdm3 in acinar cells elevated exocrine injury, enhanced immune cell activation and infiltration, and greatly increased acinar-to-ductal cell reprogramming upon cerulein-induced pancreatitis. Whole transcriptome analyses of Prdm3 knockout acini revealed that pathways involved in inflammatory response and Hif-1 signaling were significantly upregulated in Prdm3-depleted acinar cells. Taken together, our results suggest that Prdm3 favors the maintenance of acinar cell homeostasis through modulation of their response to inflammation and oncogenic Kras activation, and thus plays a previously unexpected suppressive role during PDAC initiation.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures
Similar articles
-
Krüppel-like Factor 5, Increased in Pancreatic Ductal Adenocarcinoma, Promotes Proliferation, Acinar-to-Ductal Metaplasia, Pancreatic Intraepithelial Neoplasia, and Tumor Growth in Mice.Gastroenterology. 2018 Apr;154(5):1494-1508.e13. doi: 10.1053/j.gastro.2017.12.005. Epub 2017 Dec 15. Gastroenterology. 2018. PMID: 29248441 Free PMC article.
-
miR-802 Suppresses Acinar-to-Ductal Reprogramming During Early Pancreatitis and Pancreatic Carcinogenesis.Gastroenterology. 2022 Jan;162(1):269-284. doi: 10.1053/j.gastro.2021.09.029. Epub 2021 Sep 20. Gastroenterology. 2022. PMID: 34547282
-
Oncogenic KRAS Reduces Expression of FGF21 in Acinar Cells to Promote Pancreatic Tumorigenesis in Mice on a High-Fat Diet.Gastroenterology. 2019 Nov;157(5):1413-1428.e11. doi: 10.1053/j.gastro.2019.07.030. Epub 2019 Jul 25. Gastroenterology. 2019. PMID: 31352001 Free PMC article.
-
The unfolded protein response: An emerging therapeutic target for pancreatitis and pancreatic ductal adenocarcinoma.Pancreatology. 2022 Jan;22(1):148-159. doi: 10.1016/j.pan.2021.10.007. Epub 2021 Oct 28. Pancreatology. 2022. PMID: 34774415 Review.
-
Bidirectional relationship between acute pancreatitis and pancreatic cancer.Curr Opin Gastroenterol. 2024 Sep 1;40(5):431-438. doi: 10.1097/MOG.0000000000001051. Epub 2024 Jun 25. Curr Opin Gastroenterol. 2024. PMID: 38935270 Review.
Cited by
-
The Role of Histone Modification in DNA Replication-Coupled Nucleosome Assembly and Cancer.Int J Mol Sci. 2023 Mar 3;24(5):4939. doi: 10.3390/ijms24054939. Int J Mol Sci. 2023. PMID: 36902370 Free PMC article. Review.
-
A systems-based framework to computationally describe putative transcription factors and signaling pathways regulating glycan biosynthesis.Beilstein J Org Chem. 2021 Jul 22;17:1712-1724. doi: 10.3762/bjoc.17.119. eCollection 2021. Beilstein J Org Chem. 2021. PMID: 34367349 Free PMC article.
-
Metabolic reprogramming in cancer: Mechanisms and therapeutics.MedComm (2020). 2023 Mar 27;4(2):e218. doi: 10.1002/mco2.218. eCollection 2023 Apr. MedComm (2020). 2023. PMID: 36994237 Free PMC article. Review.
-
MECOM/PRDM3 and PRDM16 Serve as Prognostic-Related Biomarkers and Are Correlated With Immune Cell Infiltration in Lung Adenocarcinoma.Front Oncol. 2022 Jan 31;12:772686. doi: 10.3389/fonc.2022.772686. eCollection 2022. Front Oncol. 2022. PMID: 35174083 Free PMC article.
-
Silencing LINC00491 Inhibits Pancreatic Cancer Progression through MiR-188-5p-induced Inhibition of ZFP91.J Cancer. 2022 Mar 21;13(6):1808-1819. doi: 10.7150/jca.65071. eCollection 2022. J Cancer. 2022. PMID: 35399733 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

