The Case for Exploiting Cross-Species Epitopes in Malaria Vaccine Design
- PMID: 32174924
- PMCID: PMC7056716
- DOI: 10.3389/fimmu.2020.00335
The Case for Exploiting Cross-Species Epitopes in Malaria Vaccine Design
Abstract
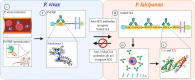
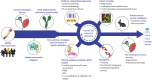
The infection dynamics between different species of Plasmodium that infect the same human host can both suppress and exacerbate disease. This could arise from inter-parasite interactions, such as competition, from immune regulation, or both. The occurrence of protective, cross-species (heterologous) immunity is an unlikely event, especially considering that strain-transcending immunity within a species is only partial despite lifelong exposure to that species. Here we review the literature in humans and animal models to identify the contexts where heterologous immunity can arise, and which antigens may be involved. From the perspective of vaccine design, understanding the mechanisms by which exposure to an antigen from one species can elicit a protective response to another species offers an alternative strategy to conventional approaches that focus on immunodominant antigens within a single species. The underlying hypothesis is that certain epitopes are conserved across evolution, in sequence or in structure, and shared in antigens from different species. Vaccines that focus on conserved epitopes may overcome the challenges posed by polymorphic immunodominant antigens; but to uncover these epitopes requires approaches that consider the evolutionary history of protein families across species. The key question for vaccinologists will be whether vaccines that express these epitopes can elicit immune responses that are functional and contribute to protection against Plasmodium parasites.
Keywords: Plasmodium; cross-species; epitopes; heterologous; immunity; malaria; vaccines.
Copyright © 2020 Mitran and Yanow.
Figures

Similar articles
-
Antibodies to Cryptic Epitopes in Distant Homologues Underpin a Mechanism of Heterologous Immunity between Plasmodium vivax PvDBP and Plasmodium falciparum VAR2CSA.mBio. 2019 Oct 8;10(5):e02343-19. doi: 10.1128/mBio.02343-19. mBio. 2019. PMID: 31594821 Free PMC article.
-
Importance of the Immunodominant CD8+ T Cell Epitope of Plasmodium berghei Circumsporozoite Protein in Parasite- and Vaccine-Induced Protection.Infect Immun. 2020 Sep 18;88(10):e00383-20. doi: 10.1128/IAI.00383-20. Print 2020 Sep 18. Infect Immun. 2020. PMID: 32719159 Free PMC article.
-
Immunization with different PfAMA1 alleles in sequence induces clonal imprint humoral responses that are similar to responses induced by the same alleles as a vaccine cocktail in rabbits.Malar J. 2011 Feb 14;10:40. doi: 10.1186/1475-2875-10-40. Malar J. 2011. PMID: 21320299 Free PMC article.
-
The immunological challenge to developing a vaccine to the blood stages of malaria parasites.Immunol Rev. 2004 Oct;201:254-67. doi: 10.1111/j.0105-2896.2004.00178.x. Immunol Rev. 2004. PMID: 15361246 Review.
-
Chimeric parasites as tools to study Plasmodium immunology and assess malaria vaccines.Methods Mol Biol. 2013;923:465-79. doi: 10.1007/978-1-62703-026-7_32. Methods Mol Biol. 2013. PMID: 22990798 Review.
Cited by
-
Plasmodium vivax malaria serological exposure markers: Assessing the degree and implications of cross-reactivity with P. knowlesi.Cell Rep Med. 2022 Jun 21;3(6):100662. doi: 10.1016/j.xcrm.2022.100662. Cell Rep Med. 2022. PMID: 35732155 Free PMC article.
-
A broadly cross-reactive i-body to AMA1 potently inhibits blood and liver stages of Plasmodium parasites.Nat Commun. 2024 Aug 22;15(1):7206. doi: 10.1038/s41467-024-50770-7. Nat Commun. 2024. PMID: 39174515 Free PMC article.
-
Five decades of clinical assessment of whole-sporozoite malaria vaccines.Front Immunol. 2022 Sep 8;13:977472. doi: 10.3389/fimmu.2022.977472. eCollection 2022. Front Immunol. 2022. PMID: 36159849 Free PMC article. Review.
-
Heterologous immunity induced by 1st generation COVID-19 vaccines and its role in developing a pan-coronavirus vaccine.Front Immunol. 2022 Aug 15;13:952229. doi: 10.3389/fimmu.2022.952229. eCollection 2022. Front Immunol. 2022. PMID: 36045689 Free PMC article. Review.
-
Editorial: Emerging talents in comparative immunology: 2022.Front Immunol. 2023 Oct 27;14:1318852. doi: 10.3389/fimmu.2023.1318852. eCollection 2023. Front Immunol. 2023. PMID: 37965318 Free PMC article. No abstract available.
References
-
- World Health Organization. World Malaria Report. Geneva: World Health Organization; (2018).
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

