Propagation of human prostate tissue from induced pluripotent stem cells
- PMID: 32170918
- PMCID: PMC7308643
- DOI: 10.1002/sctm.19-0286
Propagation of human prostate tissue from induced pluripotent stem cells
Abstract
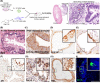
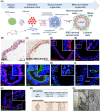
Primary culture of human prostate organoids and patient-derived xenografts is inefficient and has limited access to clinical tissues. This hampers their use for translational study to identify new treatments. To overcome this, we established a complementary approach where rapidly proliferating and easily handled induced pluripotent stem cells enabled the generation of human prostate tissue in vivo and in vitro. By using a coculture technique with inductive urogenital sinus mesenchyme, we comprehensively recapitulated in situ 3D prostate histology, and overcame limitations in the primary culture of human prostate stem, luminal and neuroendocrine cells, as well as the stromal microenvironment. This model now unlocks new opportunities to undertake translational studies of benign and malignant prostate disease.
Keywords: androgen receptor; differentiation; induced pluripotent stem cells; organoids; prostate; prostate cancer; stem cells.
© 2020 The Authors. STEM CELLS TRANSLATIONAL MEDICINE published by Wiley Periodicals, Inc. on behalf of AlphaMed Press.
Conflict of interest statement
The authors declared no potential conflicts of interest.
Figures


Similar articles
-
Human prostate organoid generation and the identification of prostate development drivers using inductive rodent tissues.Development. 2023 Jul 1;150(13):dev201328. doi: 10.1242/dev.201328. Epub 2023 Jul 12. Development. 2023. PMID: 37376888 Free PMC article.
-
A novel model of urinary tract differentiation, tissue regeneration, and disease: reprogramming human prostate and bladder cells into induced pluripotent stem cells.Eur Urol. 2013 Nov;64(5):753-61. doi: 10.1016/j.eururo.2013.03.054. Epub 2013 Apr 6. Eur Urol. 2013. PMID: 23582880 Free PMC article.
-
Estrogen-initiated transformation of prostate epithelium derived from normal human prostate stem-progenitor cells.Endocrinology. 2011 Jun;152(6):2150-63. doi: 10.1210/en.2010-1377. Epub 2011 Mar 22. Endocrinology. 2011. PMID: 21427218 Free PMC article.
-
Engineering Prostate Cancer from Induced Pluripotent Stem Cells-New Opportunities to Develop Preclinical Tools in Prostate and Prostate Cancer Studies.Int J Mol Sci. 2020 Jan 30;21(3):905. doi: 10.3390/ijms21030905. Int J Mol Sci. 2020. PMID: 32019175 Free PMC article. Review.
-
Disease Modeling Using 3D Organoids Derived from Human Induced Pluripotent Stem Cells.Int J Mol Sci. 2018 Mar 21;19(4):936. doi: 10.3390/ijms19040936. Int J Mol Sci. 2018. PMID: 29561796 Free PMC article. Review.
Cited by
-
The Sca-1+ and Sca-1- mouse prostatic luminal cell lineages are independently sustained.Stem Cells. 2020 Nov;38(11):1479-1491. doi: 10.1002/stem.3253. Epub 2020 Aug 8. Stem Cells. 2020. PMID: 32627901 Free PMC article.
-
Prostate organoids: emerging experimental tools for translational research.J Clin Invest. 2023 May 15;133(10):e169616. doi: 10.1172/JCI169616. J Clin Invest. 2023. PMID: 37183816 Free PMC article. Review.
-
Organoids: An Emerging Precision Medicine Model for Prostate Cancer Research.Int J Mol Sci. 2024 Jan 16;25(2):1093. doi: 10.3390/ijms25021093. Int J Mol Sci. 2024. PMID: 38256166 Free PMC article. Review.
-
A Review of Prostate Organogenesis and a Role for iPSC-Derived Prostate Organoids to Study Prostate Development and Disease.Int J Mol Sci. 2021 Dec 3;22(23):13097. doi: 10.3390/ijms222313097. Int J Mol Sci. 2021. PMID: 34884905 Free PMC article. Review.
-
Human prostate organoid generation and the identification of prostate development drivers using inductive rodent tissues.Development. 2023 Jul 1;150(13):dev201328. doi: 10.1242/dev.201328. Epub 2023 Jul 12. Development. 2023. PMID: 37376888 Free PMC article.
References
-
- Clevers H. Modeling development and disease with organoids. Cell. 2016;165(7):1586‐1597. - PubMed

